100491-29-0
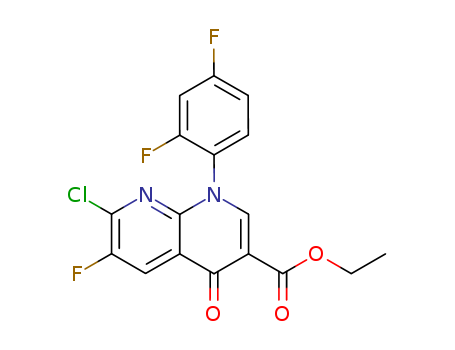
- Product Name:ETHYL 1-(2,4-DIFLUOROPHENYL)-7-CHORO-6-FLUORO-4-OXO-HYDROPYRIDINO[2,3-B] PYRIDINE-3-CARBOXYLATE
- Molecular Formula:C17H10ClF3N2O3
- Purity:99%
- Molecular Weight:382.726
Product Details
Reputable supplier selling ETHYL 1-(2,4-DIFLUOROPHENYL)-7-CHORO-6-FLUORO-4-OXO-HYDROPYRIDINO[2,3-B] PYRIDINE-3-CARBOXYLATE 100491-29-0 with stock
- Molecular Formula:C17H10ClF3N2O3
- Molecular Weight:382.726
- Appearance/Colour:almost white powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:212-215 °C
- Refractive Index:1.589
- Boiling Point:502.504 °C at 760 mmHg
- PKA:-3.23±0.70(Predicted)
- Flash Point:257.705 °C
- PSA:61.19000
- Density:1.523 g/cm3
- LogP:3.63310
ETHYL 1-(2,4-DIFLUOROPHENYL)-7-CHORO-6-FLUORO-4-OXO-HYDROPYRIDINO[2,3-B] PYRIDINE-3-CARBOXYLATE(Cas 100491-29-0) Usage
InChI:InChI=1/C17H10ClF3N2O3/c1-2-26-17(25)10-7-23(13-4-3-8(19)5-11(13)20)16-9(14(10)24)6-12(21)15(18)22-16/h3-7H,2H2,1H3
100491-29-0 Relevant articles
Method for synthesizing tosufloxacin tosilate ring compound
-
Paragraph 0022-0041, (2019/10/10)
The invention discloses a method for syn...
ANTIVIRAL AND ANTIMICROBIAL COMPOUNDS
-
Paragraph 0128; 0133, (2014/03/25)
Disclosed are guanidine and biguanidine ...
Quinolone-and naphthyridonecarboxylic acid derivatives
-
, (2008/06/13)
The invention relates to new quinolone- ...
Quinolone- and naphthyridonecarboxylic acid derivatives
-
, (2008/06/13)
Disclosed are new quinolone- and naphthy...
100491-29-0 Process route
-
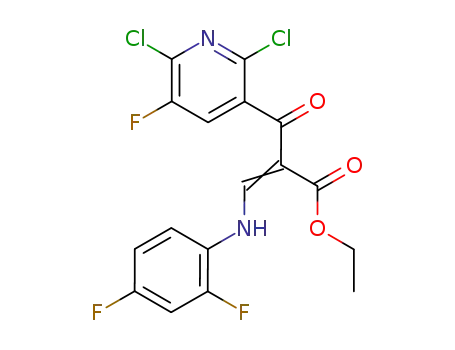
-
100490-99-1
ethyl-3,1-(2,4-difluoroanilino)-2-(2,6-dichloro-5-fluoronicotinyl) acrylate

-
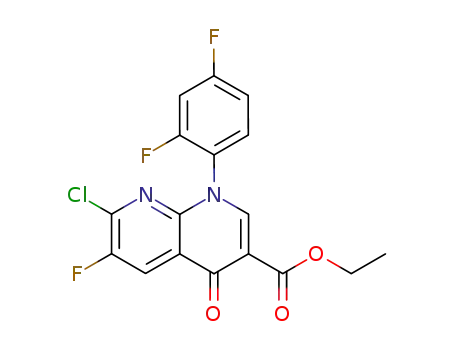
-
100491-29-0
ethyl 7-chloro-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
tetrahydrofuran;
for 1h;
Heating;
|
86.5% |
|
With
sodium hydride;
In
1,4-dioxane;
for 1h;
Ambient temperature;
|
|
|
With
sodium hydride;
In
tetrahydrofuran;
for 1.5h;
Inert atmosphere;
Reflux;
|
8 g |
|
With
sodium hydrogencarbonate;
In
dichloromethane;
at 35 ℃;
for 3h;
Temperature;
Solvent;
Reagent/catalyst;
|
-
![ethyl 2-[2-(2,4-difluorophenylamino)-5-fluoro-6-methoxynicotinoyl]acetate](/upload/2025/4/a53d319b-b262-4f1b-8276-acba5386e0b5.png)
-
105152-47-4
ethyl 2-[2-(2,4-difluorophenylamino)-5-fluoro-6-methoxynicotinoyl]acetate

-

-
100491-29-0
ethyl 7-chloro-1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate
| Conditions | Yield |
|---|---|
|
With
trichlorophosphate;
In
diethyl ether; chloroform; water; N,N-dimethyl-formamide;
|
72.2% |
100491-29-0 Upstream products
-
100490-99-1

ethyl-3,1-(2,4-difluoroanilino)-2-(2,6-dichloro-5-fluoronicotinyl) acrylate
-
96568-04-6
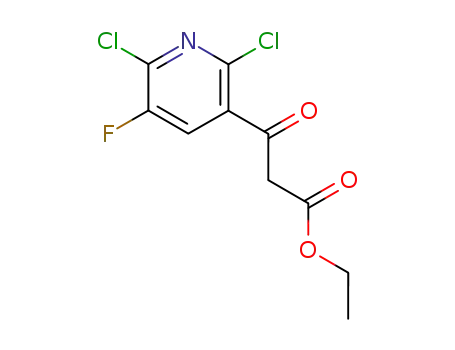
ethyl 3-(2,6-dichloro-5-fluoropyridin-3-yl)-3-oxopropanoate
-
96568-02-4
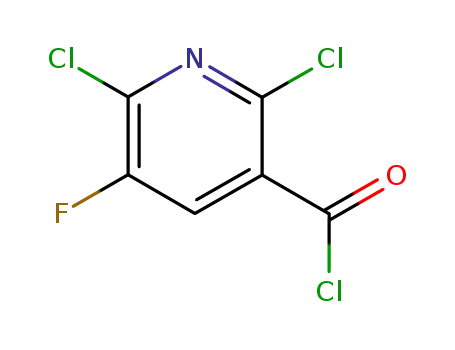
2,6-dichloro-5-fluoro-3-nicotinoyl chloride
-
82671-06-5
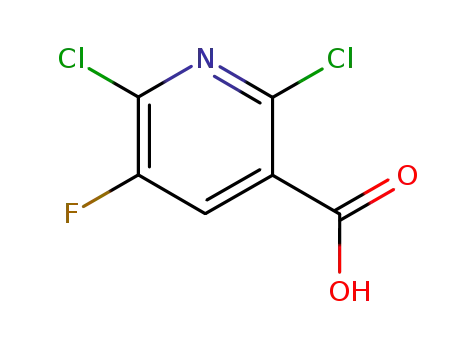
2,6-dichloro-5-fluoro-3-pyridinecarboxylic acid
100491-29-0 Downstream products
-
100491-63-2
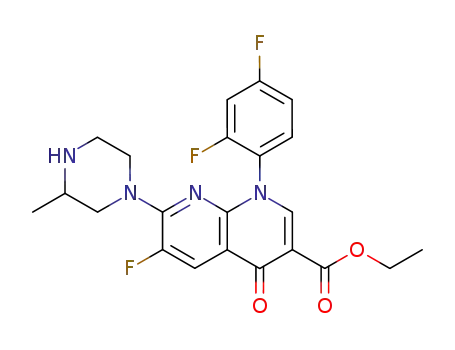
1-(2,4-Difluoro-phenyl)-6-fluoro-7-(3-methyl-piperazin-1-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid ethyl ester
-
100491-94-9
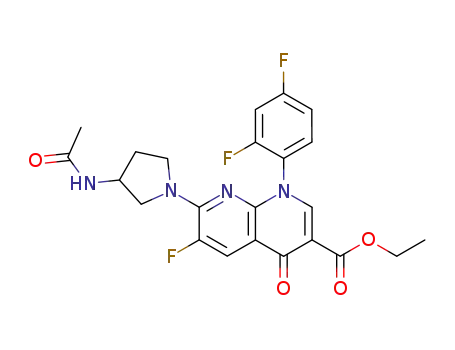
ethyl 7-(3-acetylamino-1-pyrrolidinyl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylate
-
134046-56-3
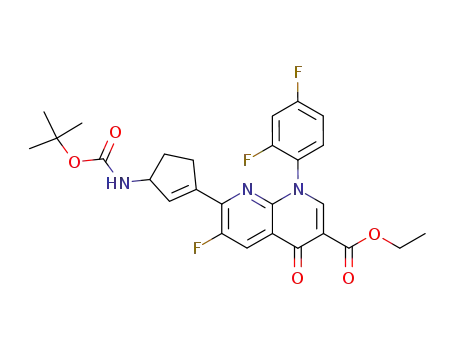
ethyl 1-(2,4-difluorophenyl)-7-<3-<<(1,1-dimethylethoxy)carbonyl>amino>-1-cyclopenten-1-yl>-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylate
-
143005-00-9
7-((1R,4R,6S)-5-Benzyl-6-methyl-2,5-diaza-bicyclo[2.2.1]hept-2-yl)-1-(2,4-difluoro-phenyl)-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid ethyl ester
Relevant Products
-
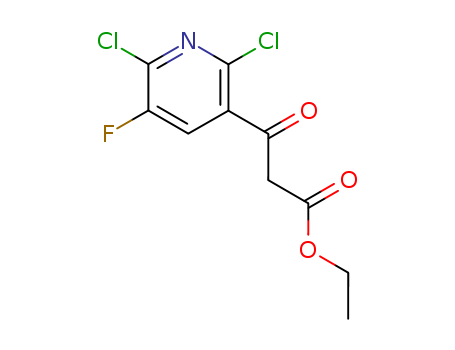
Ethyl 2,6-dichloro-5-fluoro-pyridine-3-acetoacetate
CAS:96568-04-6
-
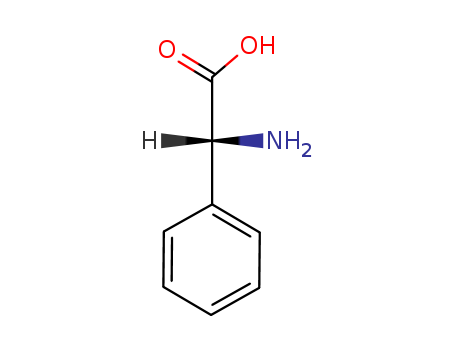
D-2-Phenylglycine
CAS:875-74-1
-
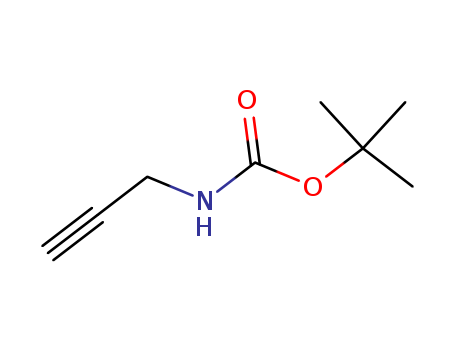
N-Boc-propargylamine
CAS:92136-39-5