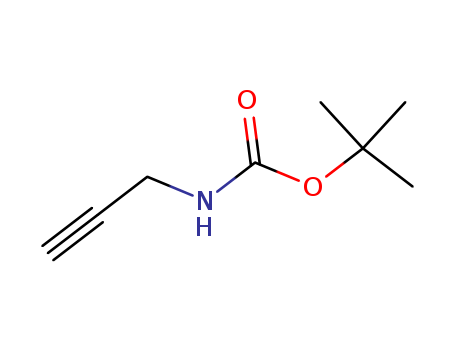
92136-39-5
- Product Name:N-Boc-propargylamine
- Molecular Formula:C8H13 N O2
- Purity:99%
- Molecular Weight:155.197
Product Details
Manufacturer supply top purity N-Boc-propargylamine 92136-39-5 with GMP standards
- Molecular Formula:C8H13 N O2
- Molecular Weight:155.197
- Vapor Pressure:0.101mmHg at 25°C
- Melting Point:40-44 ºC
- Boiling Point:222.5°Cat760mmHg
- PKA:11.24±0.46(Predicted)
- Flash Point:88.4°C
- PSA:38.33000
- Density:0.99g/cm3
- LogP:1.53520
N-Boc-propargylamine(Cas 92136-39-5) Usage
InChI:InChI=1/C8H13NO2/c1-5-6-9-7(10)11-8(2,3)4/h1H,6H2,2-4H3,(H,9,10)
92136-39-5 Relevant articles
PH-controlled aggregation polymorphism of amyloidogenic Aβ (16-22): Insights for obtaining peptide tapes and peptide nanotubes, as function of the N -terminal capping moiety
Elgersma, Ronald C.,Kroon-Batenburg, Loes M.J.,Posthuma, George,Meeldijk, Johannes D.,Rijkers, Dirk T.S.,Liskamp, Rob M.J.
, p. 55 - 65 (2014)
Peptide and protein self-assembly result...
Two Facile General Methods for the Conjugation of Three Different Molecules
Oh, Keumrok,Shin, Dong Seok,Kim, Hyeong Baik,Sirion, Uthaiwan,Chi, Dae Yoon
, p. 333 - 341 (2021)
In the quest to synthesize compounds tha...
Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis
Li, Huan,Wang, Fang,Zhu, Shengqing,Chu, Lingling
supporting information, (2022/01/20)
We describe here a Ni-catalyzed intermol...
From Synthetic Simplified Marine Metabolite Analogues to New Selective Allosteric Inhibitor of Aurora B Kinase
Juillet, Charlotte,Ermolenko, Ludmila,Boyarskaya, Dina,Baratte, Blandine,Josselin, Béatrice,Nedev, Hristo,Bach, Stéphane,Iorga, Bogdan I.,Bignon, Jér?me,Ruchaud, Sandrine,Al-Mourabit, Ali
, p. 1197 - 1219 (2021/02/05)
Significant inhibition of Aurora B was a...
Pillar[5]arene-Based Polycationic Glyco[2]rotaxanes Designed as Pseudomonas aeruginosa Antibiofilm Agents
Coenye, Tom,De Winter, Julien,Diaconu, Andrei,Fransolet, Maude,Gillon, Emilie,Imberty, Anne,Jimmidi, Ravikumar,Michiels, Carine,Mohy El Dine, Tharwat,Vincent, Stéphane P.
supporting information, p. 14728 - 14744 (2021/10/12)
Pseudomonas aeruginosa (P.A.) is a human...
92136-39-5 Process route
-
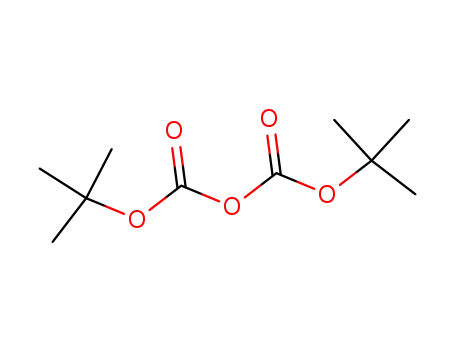
-
24424-99-5
di-tert-butyl dicarbonate

-
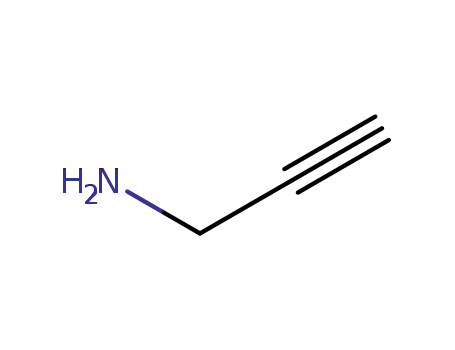
-
2450-71-7
Propargylamine

-
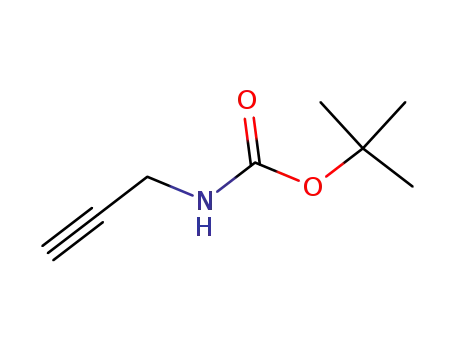
-
92136-39-5
N-tert-Butoxycarbonyl-1-amino-3-propyne
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
at 0 ℃;
|
100% |
|
In
tetrahydrofuran;
at 75 ℃;
for 1h;
|
100% |
|
With
dmap;
In
dichloromethane;
at 0 - 20 ℃;
|
100% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 18h;
|
100% |
|
In
tetrahydrofuran;
at 21 ℃;
for 4h;
Inert atmosphere;
|
100% |
|
With
triethylamine;
In
dichloromethane;
|
99.4% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
99% |
|
In
ethyl acetate;
at 0 - 20 ℃;
|
99% |
|
In
ethyl acetate;
at 20 ℃;
Cooling with ice;
|
99% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
98% |
|
In
dichloromethane;
at 0 ℃;
for 0.416667h;
|
98% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 0.5h;
|
98% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 0.5h;
Inert atmosphere;
|
98% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran;
at 25 ℃;
for 5h;
|
97% |
|
With
dmap;
In
dichloromethane;
|
97% |
|
In
water;
at 20 ℃;
|
96% |
|
With
triethylamine;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
95% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
|
95% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 3h;
Inert atmosphere;
|
95% |
|
In
tetrahydrofuran; water;
at 20 ℃;
for 48h;
|
94% |
|
In
dichloromethane;
at 20 ℃;
for 1h;
|
93% |
|
With
triethylamine;
In
methanol;
at 20 ℃;
|
93% |
|
di-tert-butyl dicarbonate; Propargylamine;
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 16h;
Inert atmosphere;
Schlenk technique;
With
1H-imidazole;
In
tetrahydrofuran; water;
at 20 ℃;
for 3h;
Inert atmosphere;
Schlenk technique;
|
93% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
92% |
|
With
dmap;
In
1,4-dioxane;
at 20 ℃;
for 48h;
|
92% |
|
With
fipronilβ-cyclodextrin;
In
methanol; acetone;
at 20 ℃;
for 0.166667h;
|
91% |
|
In
tetrahydrofuran;
at 20 ℃;
|
91% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
at 0 ℃;
for 5h;
|
90% |
|
With
triethylamine;
In
diethyl ether;
at 20 ℃;
|
90% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
|
90% |
|
In
tetrahydrofuran;
at 20 ℃;
for 4h;
Inert atmosphere;
|
90% |
|
Propargylamine;
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 0.25h;
di-tert-butyl dicarbonate;
In
dichloromethane;
|
90% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 16h;
|
90% |
|
In
dichloromethane;
for 12h;
|
90% |
|
In
dichloromethane;
for 12h;
|
90% |
|
In
dichloromethane;
for 12h;
|
90% |
|
In
tetrahydrofuran;
at 75 ℃;
for 1h;
|
89% |
|
With
sodium hydrogencarbonate;
In
chloroform;
at 25 ℃;
for 3h;
|
87% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 3.5h;
|
86% |
|
In
dichloromethane;
for 2h;
Ambient temperature;
|
84% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
84% |
|
With
sodium hydroxide; tetrabutylammomium bromide;
In
various solvent(s);
at 25 ℃;
for 1h;
|
83% |
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
|
83.9% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 2.5h;
|
82% |
|
With
sodium hydrogencarbonate;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
81% |
|
In
dichloromethane;
for 2h;
Inert atmosphere;
|
80% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 1h;
|
79% |
|
With
dmap;
In
dichloromethane;
at 20 ℃;
Cooling with ice;
|
78% |
|
With
di-tert-butyl dicarbonate; sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
for 4h;
|
75% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 1.5h;
|
75% |
|
In
tert-butyl methyl ether;
at 23 - 28 ℃;
for 2h;
|
74% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 3h;
|
72% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 3h;
|
72% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 16h;
Cooling with ice;
|
71% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 16h;
Cooling with ice;
|
71% |
|
In
dichloromethane;
at 0 ℃;
for 1h;
|
71.9% |
|
In
tetrahydrofuran;
at 0 ℃;
for 12h;
|
70% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
|
67% |
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 2.5h;
|
67.6% |
|
With
sodium hydrogencarbonate;
In
water;
|
65% |
|
In
dichloromethane;
at 20 ℃;
for 1.5h;
|
58% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 48h;
Inert atmosphere;
|
56% |
|
With
dmap;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
55% |
|
In
tetrahydrofuran;
for 12h;
|
18% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 3.25h;
|
17% |
|
With
sodium hydroxide;
In
1,4-dioxane;
|
|
|
With
triethylamine;
In
dichloromethane;
for 2h;
|
|
|
In
dichloromethane;
|
|
|
In
dichloromethane;
|
|
|
In
dichloromethane;
|
|
|
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
In
dichloromethane;
at 20 ℃;
for 1.5h;
|
|
|
With
triethylamine;
In
diethyl ether;
at 0 - 20 ℃;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
for 3h;
|
|
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
dmap;
In
dichloromethane;
at 20 ℃;
|
|
|
In
dichloromethane;
at 20 ℃;
for 3h;
|
|
|
With
N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 20 ℃;
for 18h;
|
|
|
In
dichloromethane;
at 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
water; sodium hydrogencarbonate;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
|
|
|
In
methanol;
at 0 - 20 ℃;
for 1h;
|
|
|
In
dichloromethane;
at 0 ℃;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 5h;
|
|
|
With
triethylamine;
|
|
|
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
4.1 g |
|
In
dichloromethane;
|
|
|
In
tetrahydrofuran;
at 80 ℃;
for 1h;
|
|
|
With
piperidine;
at 0 - 20 ℃;
for 3h;
|
|
|
With
triethylamine;
In
tetrahydrofuran; water;
at 20 ℃;
|
-

-
24424-99-5
di-tert-butyl dicarbonate

-
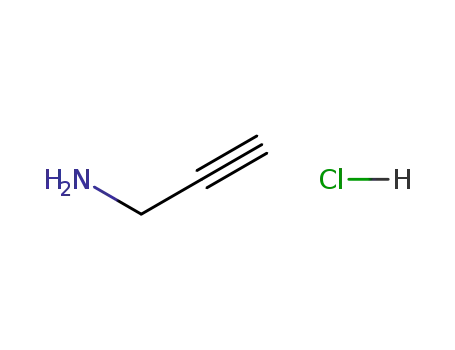
-
15430-52-1
prop-2-yn-1-amine hydrochloride

-

-
92136-39-5
N-tert-Butoxycarbonyl-1-amino-3-propyne
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
tetrahydrofuran;
for 1h;
|
99% |
|
With
triethylamine;
In
dichloromethane;
for 2h;
Cooling with ice;
|
96% |
|
With
triethylamine;
In
dichloromethane;
for 6h;
|
80% |
92136-39-5 Upstream products
-
24424-99-5

di-tert-butyl dicarbonate
-
2450-71-7

Propargylamine
-
90965-06-3
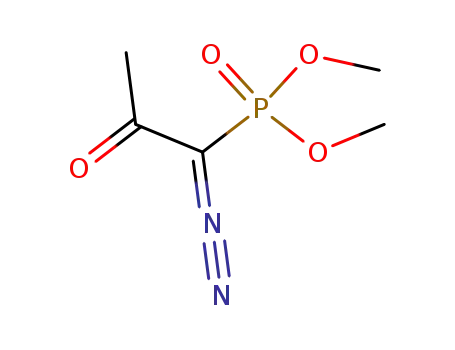
dimethyl 1-(1-diazo-2-oxopropyl)phosphonate
-
121505-93-9
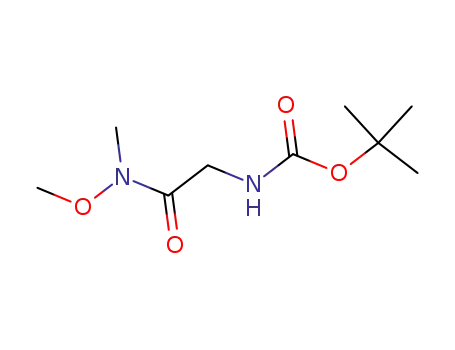
tert-butyl N-{[methoxy(methyl)carbamoyl]methyl}carbamate
92136-39-5 Downstream products
-
92136-43-1
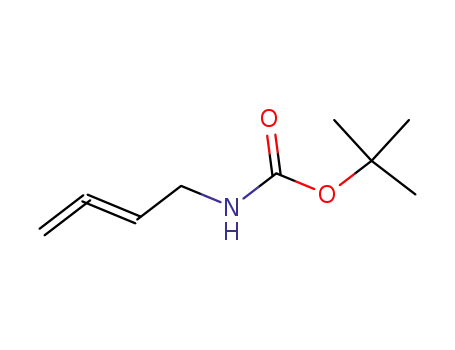
tert-butyl 2,3-butadienylcarbamic acid
-
154016-57-6
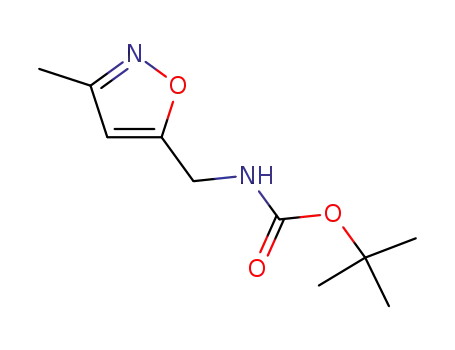
tert-butyl (3-methylisoxazol-5-yl)methylcarbamate
-
147528-20-9
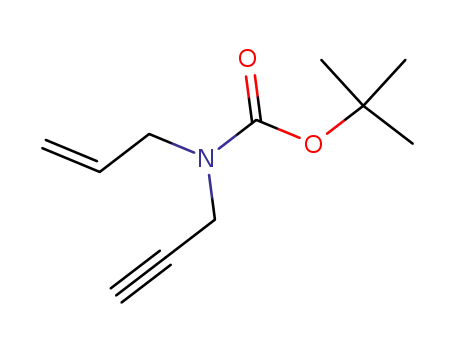
N-(tert-butoxycarbonyl)-N-allyl-3-amino-1-propyne
-
214749-90-3
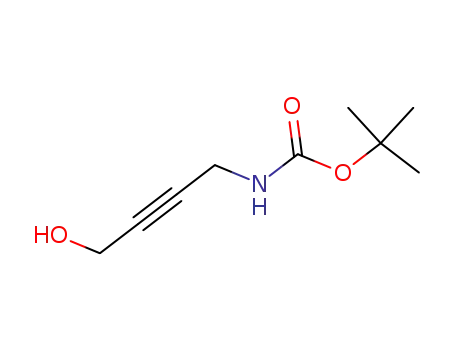
1-(tert-butoxycarbonylamino)-4-hydroxy-2-butyne
Relevant Products
-
4-ACETAMIDO-TEMPO
CAS:14691-89-5
-
2,6-Dichloro-5-fluoro-3-pyridinecarbonitrile
CAS:82671-02-1