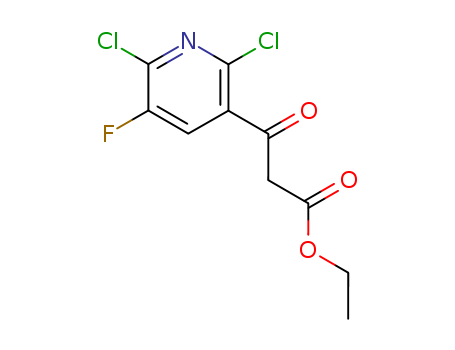
96568-04-6
- Product Name:Ethyl 2,6-dichloro-5-fluoro-pyridine-3-acetoacetate
- Molecular Formula:C10H8Cl2FNO3
- Purity:99%
- Molecular Weight:280.083
Product Details
Quality manufacturer supply Ethyl 2,6-dichloro-5-fluoro-pyridine-3-acetoacetate 96568-04-6 in stock with high standard
- Molecular Formula:C10H8Cl2FNO3
- Molecular Weight:280.083
- Appearance/Colour:Off-white powder
- Vapor Pressure:4.57E-05mmHg at 25°C
- Melting Point:68-72 °C(lit.)
- Refractive Index:1.524
- Boiling Point:349.9 °C at 760 mmHg
- PKA:9.43±0.50(Predicted)
- Flash Point:165.4 °C
- PSA:56.26000
- Density:1.434 g/cm3
- LogP:2.59230
Ethyl 2,6-dichloro-5-fluoro-pyridine-3-acetoacetate(Cas 96568-04-6) Usage
|
General Description |
Ethyl 2,6-dichloro-5-fluoro-β-oxo-3-pyridinepropionate is a β-keto ester. |
InChI:InChI=1/C10H8Cl2FNO3/c1-2-17-8(16)4-7(15)5-3-6(13)10(12)14-9(5)11/h3H,2,4H2,1H3
96568-04-6 Relevant articles
Synthesis, antimycobacterial and antibacterial activity of 1-(6-amino-3,5-difluoropyridin-2-yl)fluoroquinolone derivatives containing an oxime functional moiety
Huang, Ju,Wang, Minghua,Wang, Bin,Wu, Zhaoyang,Liu, Mingliang,Feng, Lianshun,Zhang, Jun,Li, Xiaoning,Yang, Yang,Lu, Yu
supporting information, p. 2262 - 2267 (2016/04/20)
A series of novel 1-(6-amino-3,5-difluor...
ANTIVIRAL AND ANTIMICROBIAL COMPOUNDS
-
, (2014/03/25)
Disclosed are guanidine and biguanidine ...
1,8-Naphthyridine-3-carboxamide derivatives with anticancer and anti-inflammatory activity
Kumar, Vivek,Jaggi, Manu,Singh, Anu T.,Madaan, Alka,Sanna, Vinod,Singh, Pratibha,Sharma, Pramod K.,Irchhaiya, Raghuveer,Burman, Anand C.
body text, p. 3356 - 3362 (2009/10/23)
A number of 1-propargyl-1,8-naphthyridin...
Process for Preparing Beta-Ketoester Compounds
-
Page/Page column 3, (2008/12/08)
The present invention relates to a proce...
96568-04-6 Process route
-
-
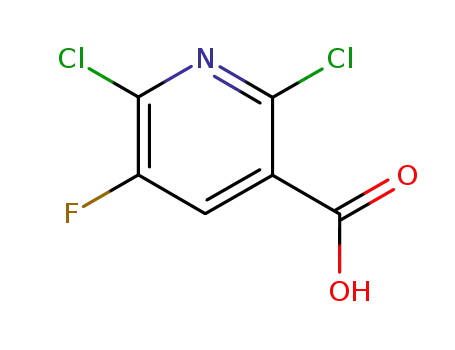
82671-06-5
2,6-dichloro-5-fluoro-3-pyridinecarboxylic acid

-
-
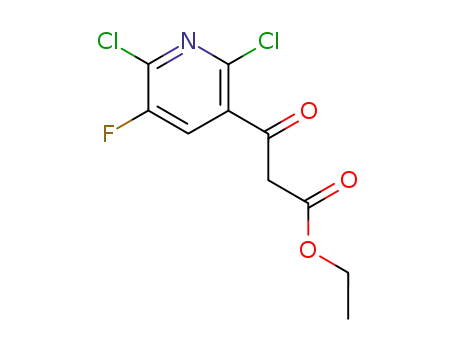
96568-04-6
ethyl 3-(2,6-dichloro-5-fluoropyridin-3-yl)-3-oxopropanoate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: SOCl2, DMF / toluene / 3 h / Heating
2: 99 percent / MgCl2, 2-methylimidazole / tetrahydrofuran / 1.) 22 deg C, 20 min; 10 deg C, 20 min, 2.) r.t., 18 h
3: 70 percent / pyridinium tosylate / ethanol / 18 h / Heating
With
thionyl chloride; 2-methylimidazole; pyridinium p-toluenesulfonate; N,N-dimethyl-formamide; magnesium chloride;
In
tetrahydrofuran; ethanol; toluene;
|
|
|
Multi-step reaction with 2 steps
1: thionyl chloride / 2 h / 80 °C
2: 1.) n-BuLi, biquinoline / 1.) THF, hexane, from -30 deg C to -5 deg C, 2.) THF, hexane, RT, 1 h
With
n-butyllithium; thionyl chloride; biquinoline;
|
|
|
Multi-step reaction with 2 steps
1: PCl5 / 0.13 h / 100 °C
2: 2.) p-TsOH*H2O / 1.) ether, RT, 2 h, 2.) H2O, reflux, 2.5 h
With
phosphorus pentachloride; toluene-4-sulfonic acid;
|
|
|
Multi-step reaction with 3 steps
1: 81 percent / SOCl2 / 1 h / Heating
2: 1.) Mg, CCl4, EtOH / 1.) Et2O, reflux, 30 min, 2.) 10 min
3: p-toluenesulfonic acid / H2O / 2 h / Heating
With
tetrachloromethane; thionyl chloride; ethanol; toluene-4-sulfonic acid; magnesium;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: thionyl chloride / 2.5 h / 85 °C
2: 2,2'-biquinoline; n-butyllithium / tetrahydrofuran; hexane / 1 h / -50 - 20 °C
With
n-butyllithium; thionyl chloride; 2,2'-biquinoline;
In
tetrahydrofuran; hexane;
|
|
|
Multi-step reaction with 3 steps
1.1: thionyl chloride / N,N-dimethyl-formamide / 4 h / Reflux
2.1: magnesium / tetrachloromethane; ethanol / 3.25 h / Reflux
2.2: 2.33 h / -10 °C
3.1: toluene-4-sulfonic acid / water / 5 h / Reflux
With
thionyl chloride; toluene-4-sulfonic acid; magnesium;
In
tetrachloromethane; ethanol; water; N,N-dimethyl-formamide;
|
-
-
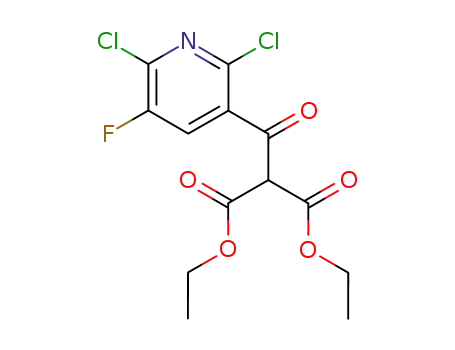
96568-03-5
diethyl (2,6-dichloro-5-fluoro-pyridine-3-carbonyl)-malonate

-

-
96568-04-6
ethyl 3-(2,6-dichloro-5-fluoropyridin-3-yl)-3-oxopropanoate
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
In
water;
for 2h;
Yield given;
Heating;
|
|
|
With
water;
toluene-4-sulfonic acid;
at 100 ℃;
for 1h;
|
|
|
With
toluene-4-sulfonic acid;
In
water;
for 5h;
Reflux;
|
96568-04-6 Upstream products
-
1071-46-1
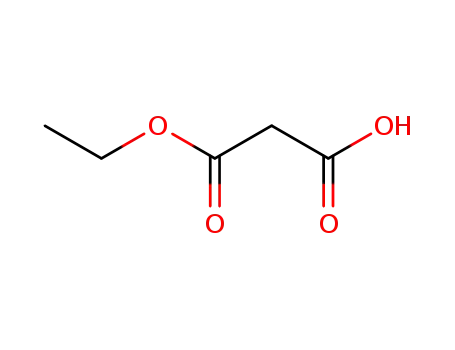
hydrogen ethyl malonate
-
96568-02-4
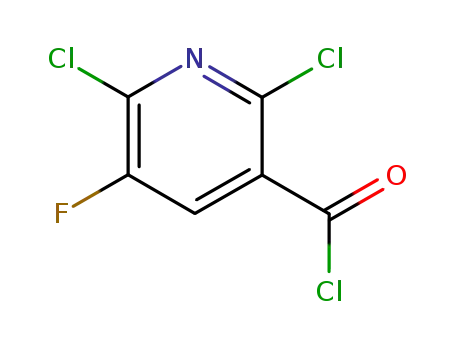
2,6-dichloro-5-fluoro-3-nicotinoyl chloride
-
35227-78-2
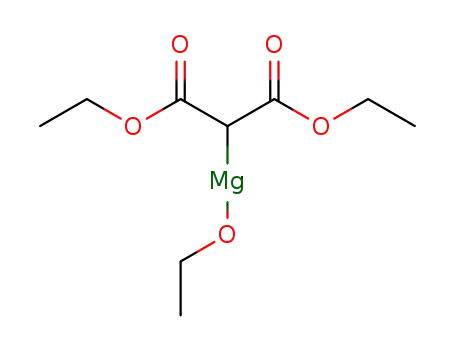
diethyl (ethoxymagnesio)malonate
-
6148-64-7
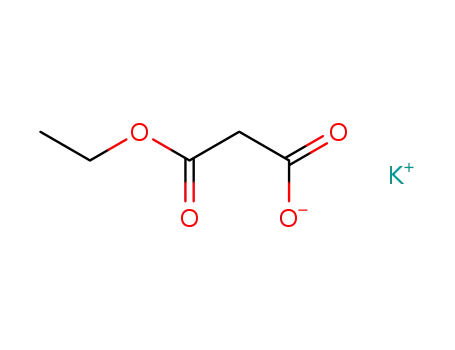
ethyl potassium malonate
96568-04-6 Downstream products
-
130878-52-3
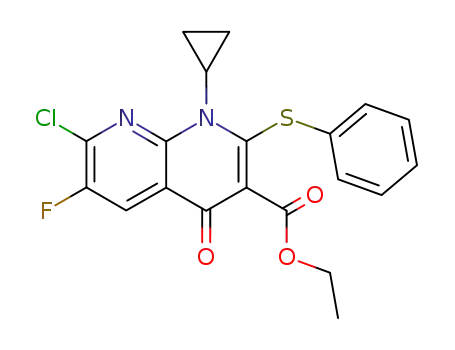
Ethyl 1-cyclopropyl-2-phenylthio-6-fluoro-7-chloro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylate
-
100490-99-1
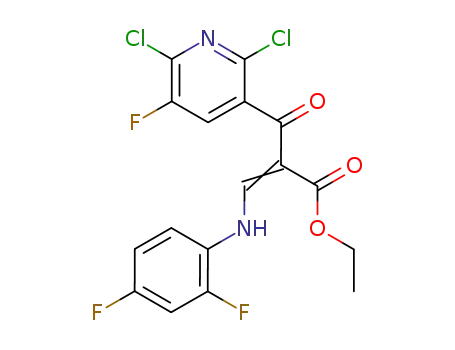
ethyl-3,1-(2,4-difluoroanilino)-2-(2,6-dichloro-5-fluoronicotinyl) acrylate
-
100426-73-1
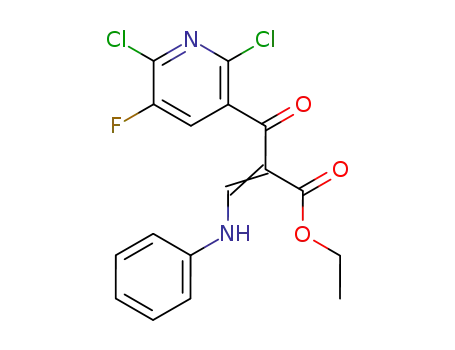
(Z)-2-(2,6-Dichloro-5-fluoro-pyridine-3-carbonyl)-3-phenylamino-acrylic acid ethyl ester
-
100361-18-0
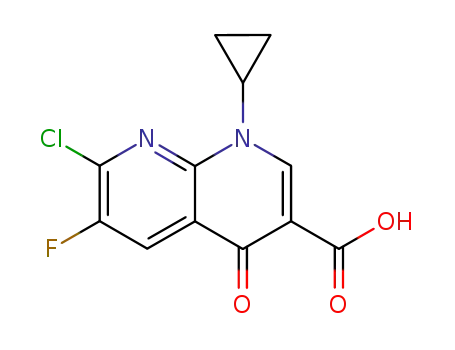
7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid
Relevant Products
-
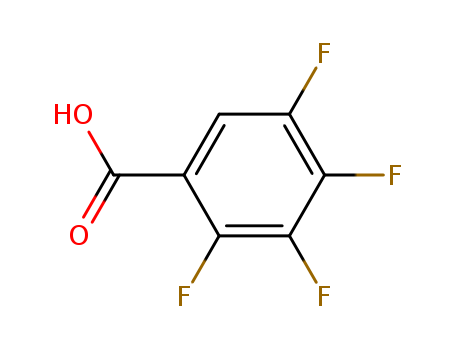
2,3,4,5-Tetrafluorobenzoic acid
CAS:1201-31-6
-
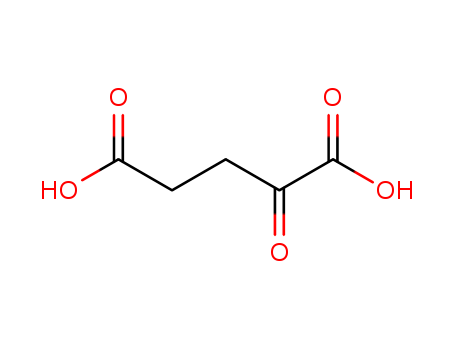
2-Ketoglutaric acid
CAS:328-50-7