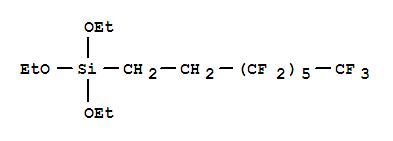
1201-31-6
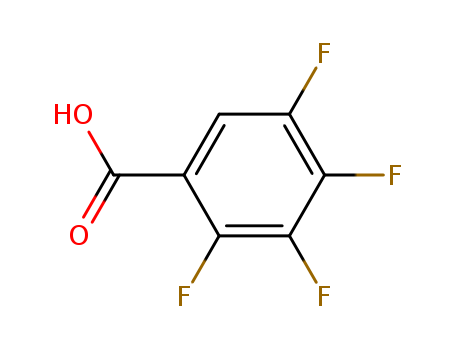
- Product Name:2,3,4,5-Tetrafluorobenzoic acid
- Molecular Formula:C7H2F4O2
- Purity:99%
- Molecular Weight:194.085
Product Details
Factory Sells Best Quality 2,3,4,5-Tetrafluorobenzoic acid 1201-31-6 with USP
- Molecular Formula:C7H2F4O2
- Molecular Weight:194.085
- Appearance/Colour:white to light yellow crystal powder
- Melting Point:85-87 °C(lit.)
- Boiling Point:239.2 °C at 760 mmHg
- PKA:2.53±0.10(Predicted)
- Flash Point:98.5 °C
- PSA:37.30000
- Density:1.633 g/cm3
- LogP:1.94120
2,3,4,5-Tetrafluorobenzoic acid(Cas 1201-31-6) Usage
InChI:InChI=1/C9H12N4O.CHF3O3S/c1-10-3-5-12(7-10)9(14)13-6-4-11(2)8-13;2-1(3,4)8(5,6)7/h3-8H,1-2H3;(H,5,6,7)/q+2;/p-1
1201-31-6 Relevant articles
-
Tamborski,C.,Soloski,E.J.
, p. 385 - 391 (1967)
-
Decarboxylation of tetrafluorophthalic acid in NH3-enriched high temperature liquid water
Fu, Jie,Mo, Jing,Zhang, Jing,Lu, Xiuyang
, p. 10 - 15 (2016)
2,3,4,5-Tetrafluorobenzoic acid is an im...
Regiospecific Replacement of Fluorine by Hydrogen in an Aromatic Ring Induced by a Rare Earth Organometallic
Deacon, Glen B.,Forsyth, Craig M.,Sun, Junhui
, p. 1095 - 1098 (1994)
Pentafluorobenzoic acid reacts with YbCp...
Preparation of 2,3,4,5-tetrafluorobenzoic acid
Li, Hua,Wang, Hongkai,Zhao, Ruiju,Liu, Juan,Zhao, Zhengui,Hu, Guoqin,Liang, Zhengyong
, p. 744 - 748 (2010)
2,3,4,5-Tetrafluorobenzoic acid, an impo...
Regioselective ortho-hydrodefluorination of pentafluorobenzoic acid by low-valent nickel complexes
Adonin,Starichenko
, p. 65 - 67 (2000)
2,3,4,5-Tetrafluorobenzoic and 3,4,5-tri...
Elemental fluorine. Part 1. Synthesis of fluoroaromatic compounds
Chambers, Richard D.,Skinner, Christopher J.,Hutchinson, John,Thomson, Julie
, p. 605 - 609 (1996)
Direct fluorination of 4-fluorobenzoic a...
Technological method for preparing 2, 3, 4, 5-tetrachloride phthalic anhydride
-
Paragraph 0039-0044; 0061, (2018/05/01)
The invention relates to an improved tec...
Method for preparing 2,3,4,5-tetrafluoro benzoic acid and 1,2,3,4-tetrafluorobenzene
-
Paragraph 0039; 0040, (2016/11/28)
The present invention discloses a method...
Gold(I)-catalyzed protodecarboxylation of (Hetero)aromatic carboxylic acids
Dupuy, Stéphanie,Nolan, Steven P.
supporting information, p. 14034 - 14038 (2013/11/19)
Readily available, inexpensive and easy ...
1201-31-6 Process route
-
-
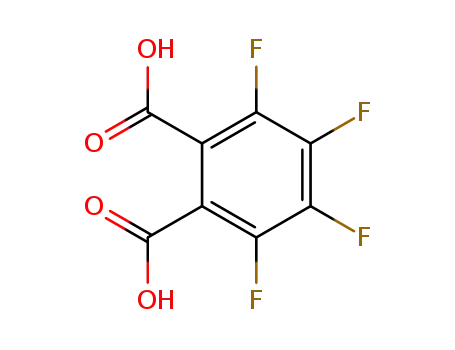
652-03-9
3,4,5,6-tetrafluorophthalic acid

-
-
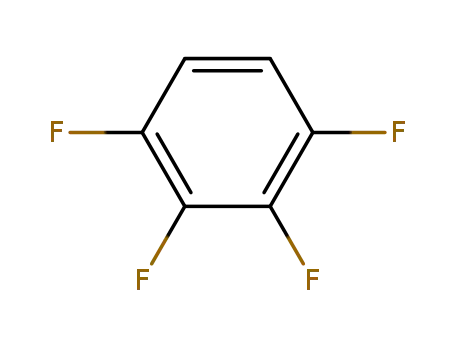
551-62-2
1,2,3,4-tetrafluorobenzene

-
-
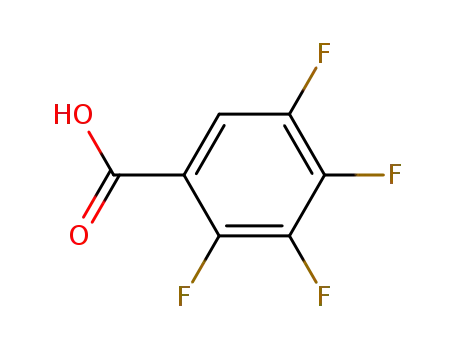
1201-31-6
2,3,4,5-tetrafluorobenzoic acid
| Conditions | Yield |
|---|---|
|
With
ammonium hydroxide;
at 190 ℃;
for 2h;
Temperature;
Time;
Autoclave;
Green chemistry;
|
86.1% 10.5% |
|
With
ammonium hydroxide;
at 240 ℃;
for 0.666667h;
Temperature;
Time;
Autoclave;
Green chemistry;
|
82% 8.1% |
-
-
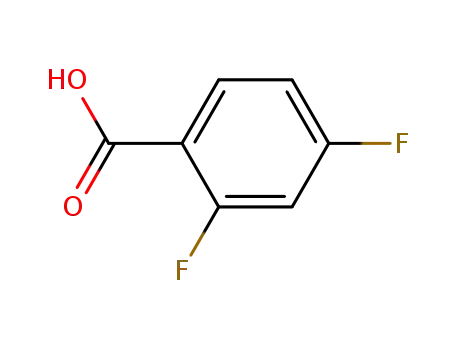
1583-58-0
2,4-difluoro-benzoic acid

-

-
1201-31-6
2,3,4,5-tetrafluorobenzoic acid

-
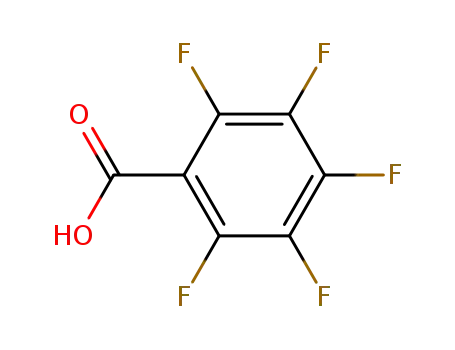
-
602-94-8
Pentafluorobenzoic acid

-
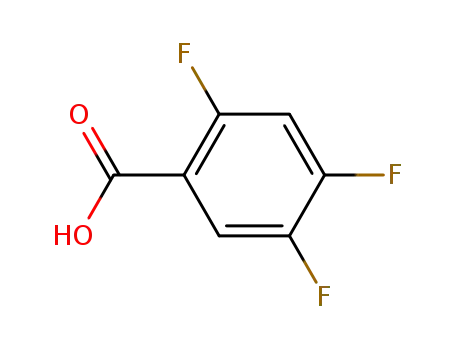
-
446-17-3
2,4,5-trifluorobenzoic acid

-
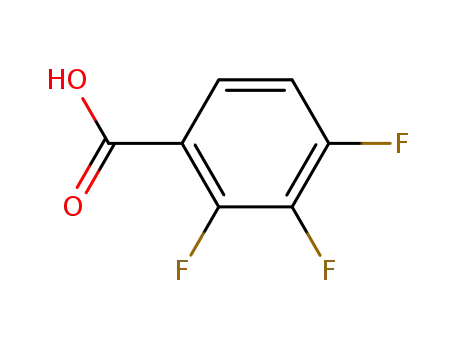
-
61079-72-9
2,3,4,-trifluorobenzoic acid
| Conditions | Yield |
|---|---|
|
With
fluorine;
In
sulfuric acid;
Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
1201-31-6 Upstream products
-
16583-06-5
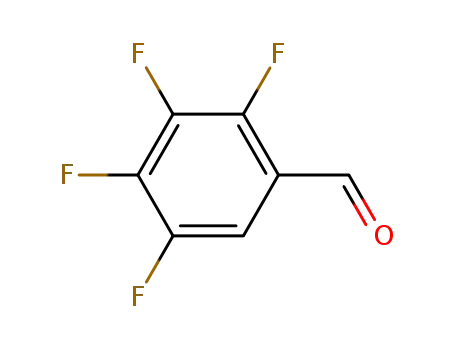
2,3,4,5-tetrafluorobenzaldehyde
-
21622-20-8
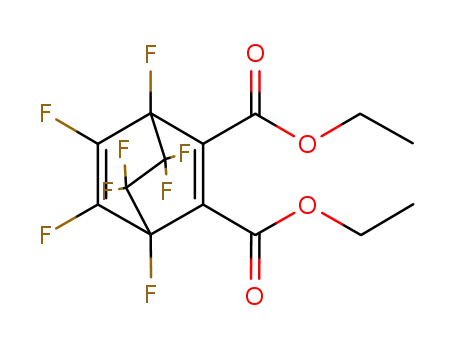
2,3-Bis-
-1,4,5,6,7,7,8,8-octafluor-bicyclo<2.2.2>octadien-(2,5) -
16582-94-8
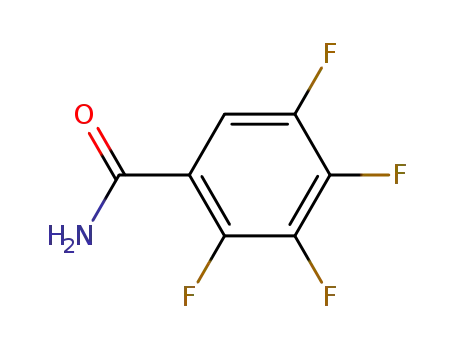
2,3,4,5-tetrafluorobenzamide
-
124-38-9

carbon dioxide
1201-31-6 Downstream products
-
16583-08-7
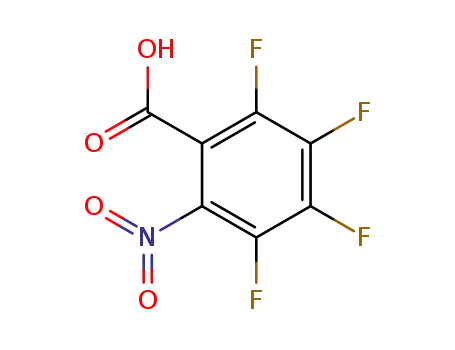
2,3,4,5-tetrafluoro-6-nitrobenzoic acid
-
94695-48-4
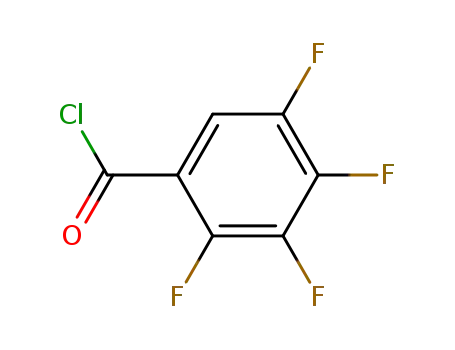
2,3,4,5-tetrafluorobenzoyl chloride
-
16583-04-3
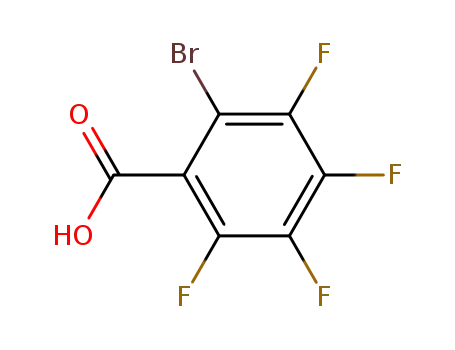
2-bromo-3,4,5,6-tetrafluorobenzoic acid
-
122894-73-9
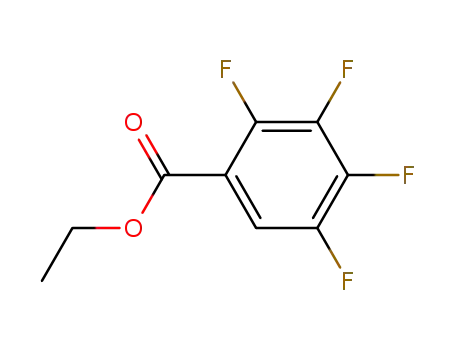
ethyl 2,3,4,5-tetrafluorobenzenecarboxylate
Relevant Products
-
1H,1H,2H,2H-Perfluorooctyltriethoxysilane
CAS:51851-37-7
-
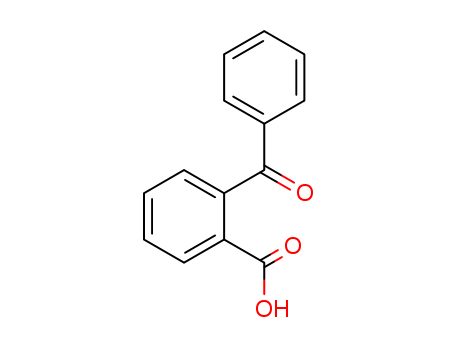
2-Benzoylbenzoic acid
CAS:85-52-9
-
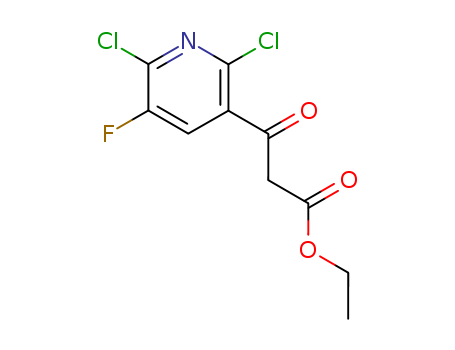
Ethyl 2,6-dichloro-5-fluoro-pyridine-3-acetoacetate
CAS:96568-04-6