875-74-1
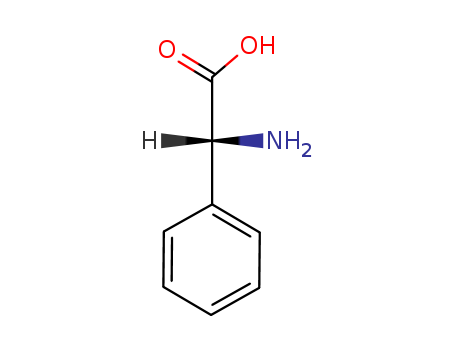
- Product Name:D-2-Phenylglycine
- Molecular Formula:C8H9NO2
- Purity:99%
- Molecular Weight:151.165
Product Details
Quality Factory Sells Top Purity 99% D-2-Phenylglycine 875-74-1 with Safe Delivery
- Molecular Formula:C8H9NO2
- Molecular Weight:151.165
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0.00107mmHg at 25°C
- Melting Point:302 °C (dec.)(lit.)
- Refractive Index:-158 ° (C=1, 1mol/L HCl)
- Boiling Point:288.7 °C at 760 mmHg
- PKA:1.94±0.10(Predicted)
- Flash Point:128.4 °C
- PSA:63.32000
- Density:1.246 g/cm3
- LogP:1.47130
D-2-Phenylglycine(Cas 875-74-1) Usage
|
Storage |
Store at room temperature. Store in cool dry place in tightly closed container. Store away from oxidizing agent. |
|
Flammability and Explosibility |
Nonflammable |
|
Definition |
ChEBI: The R stereoisomer of alpha-phenylglycine. |
InChI:InChI=1/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m1/s1
875-74-1 Relevant articles
Formation of Quasi-racemic Diastereoisomeric Salts as a Structural Cause for Efficient Optical Resolution
Fogassy, Elemer,Kozma, David
, p. 5069 - 5070 (1995)
During optical resolutions, when the res...
-
Timmermans,Motiuk
, p. 399,408, 410 (1932)
-
The Methyl Ester of α-Aminophenylacetic Acid: pH-Dependence and Phosphate Catalysis of Hydrolysis
Blinkovsky, Alexander M.,Galaev, Igor Yu.,Svedas, Vytas K.
, p. 1537 - 1540 (1986)
The dependence of the rate of spontaneou...
Highly Stable Zr(IV)-Based Metal-Organic Frameworks for Chiral Separation in Reversed-Phase Liquid Chromatography
Jiang, Hong,Yang, Kuiwei,Zhao, Xiangxiang,Zhang, Wenqiang,Liu, Yan,Jiang, Jianwen,Cui, Yong
supporting information, p. 390 - 398 (2021/01/13)
Separation of racemic mixtures is of gre...
D-Phenylglycine aminotransferase (d-PhgAT)-substrate scope and structural insights of a stereo-inverting biocatalyst used in the preparation of aromatic amino acids
Akhtar, M. Kalim,Campopiano, Dominic J.,De Cesare, Silvia,Loake, Gary J.,Marles-Wright, Jon,Serpico, Annabel
, p. 6533 - 6543 (2020/11/13)
Enantiopure amines are key building bloc...
Ultrasound-Controlled Chiral Separation of Four Amino Acids and 2,2,2-Trifluoro-1-(9-anthryl)ethanol
Lee, Jae Hwan,Ryoo, Jae Jeong
, p. 146 - 149 (2019/02/07)
Chiral separation of 4-hydroxyphenylglyc...
Deracemization and stereoinversion to aromatic d-amino acid derivatives with ancestral l-amino acid oxidase
Nakano, Shogo,Minamino, Yuki,Hasebe, Fumihito,Ito, Sohei
, p. 10152 - 10158 (2019/10/19)
Enantiomerically pure amino acid derivat...
875-74-1 Process route
-
-
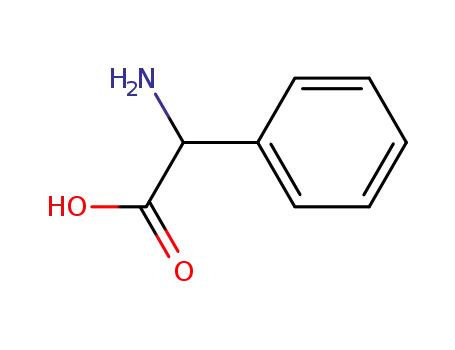
2835-06-5
phenylglycin

-
-
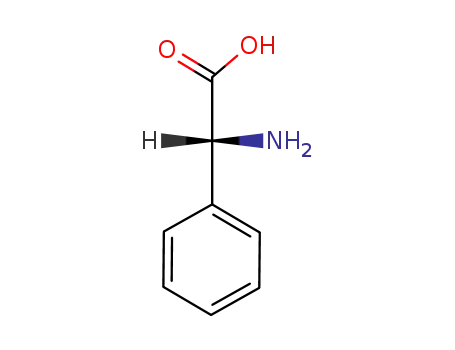
875-74-1
(R)-phenylglycine

-
-
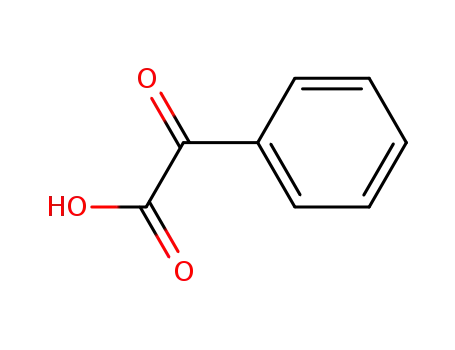
611-73-4
Benzoylformic acid

-
-
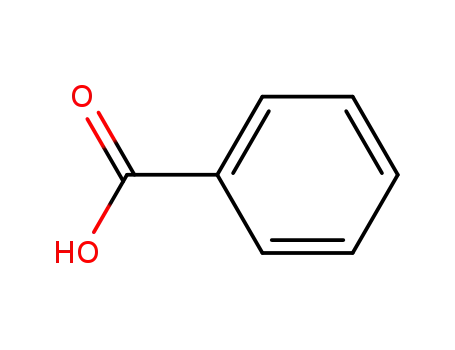
65-85-0,8013-63-6
benzoic acid
| Conditions | Yield |
|---|---|
|
|
-
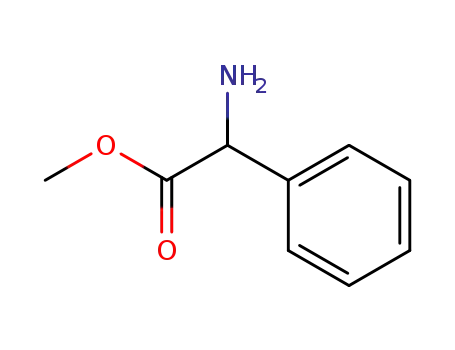
-
24461-61-8,26682-99-5,37760-98-8,6591-61-3
phenylglycine methyl ester

-
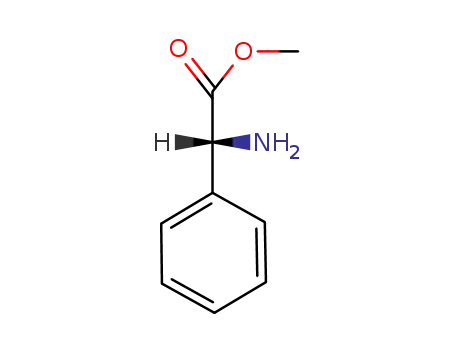
-
24461-61-8
(R)-Phenylglycine methyl ester

-
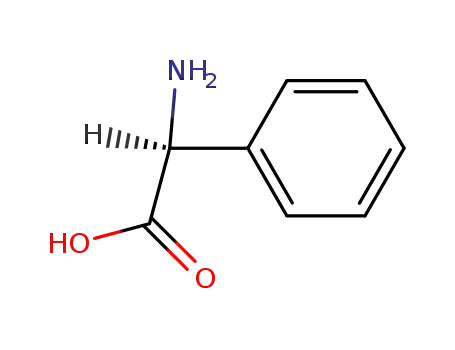
-
2935-35-5
(S)-2-phenylglycine

-

-
875-74-1
(R)-phenylglycine

-
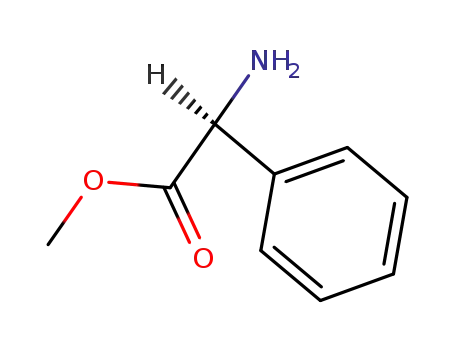
-
6591-61-3,24461-61-8,26682-99-5,37760-98-8
(S)-Phenylglycine methyl ester
| Conditions | Yield |
|---|---|
|
With
Bacillus licheniformis; sodium hydrogencarbonate; 1-ethyl-3-methylimidazolium acetate;
In
water; water-d2;
at 30 ℃;
for 24h;
|
875-74-1 Upstream products
-
4755-72-0
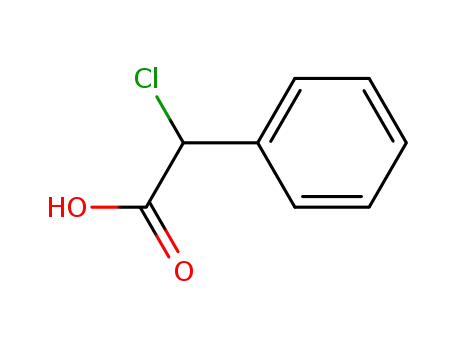
Chloro-phenyl-acetic acid
-
700-63-0
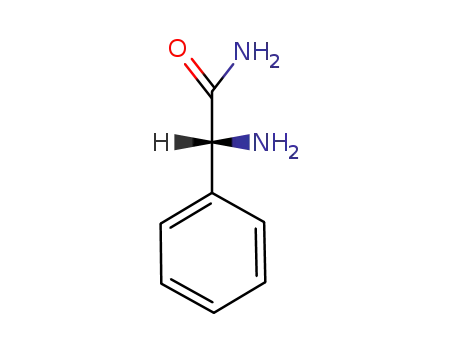
(R)-phenylglycine amide
-
10419-67-7
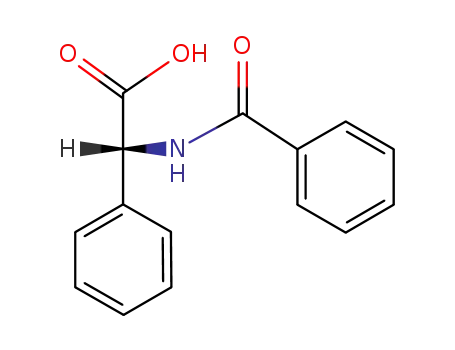
N-[(2S)-(benzoylamino)(phenyl)]ethanoic acid
-
60686-79-5
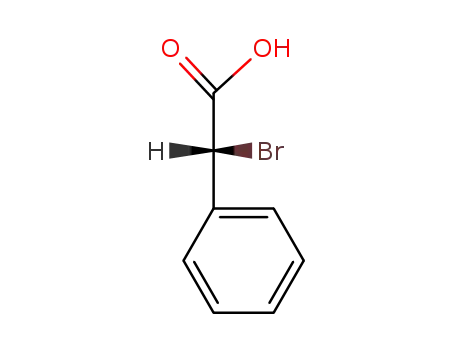
(R)-2-bromo-2-phenylacetic acid
875-74-1 Downstream products
-
24461-61-8

(R)-Phenylglycine methyl ester
-
17609-48-2
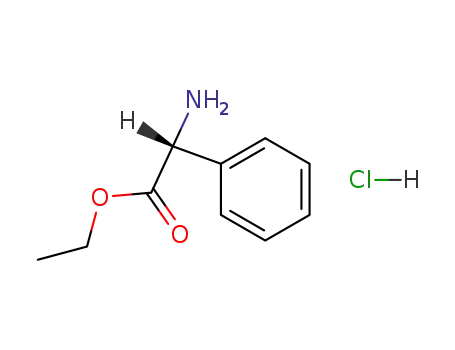
(R)-2-phenylglycine ethyl ester hydrochloride
-
39251-40-6
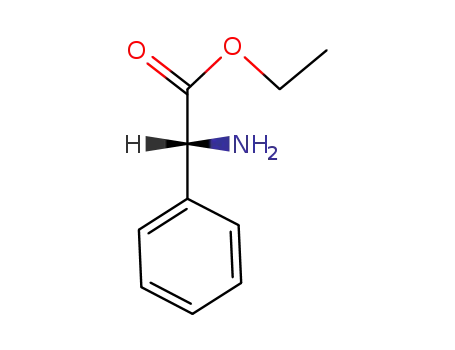
(R)-amino-phenyl-acetic acid ethyl ester
-
14328-52-0
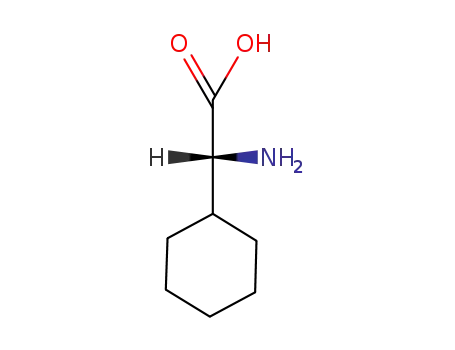
(R)-2-amino-2-cyclohexyl acetic acid
Relevant Products
-
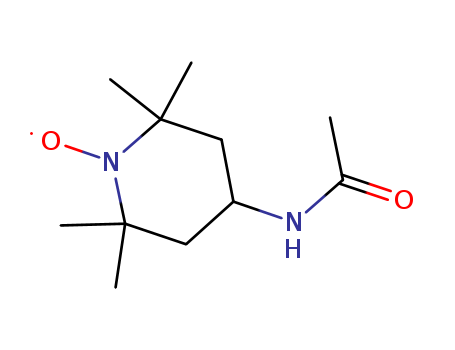
4-ACETAMIDO-TEMPO
CAS:14691-89-5
-
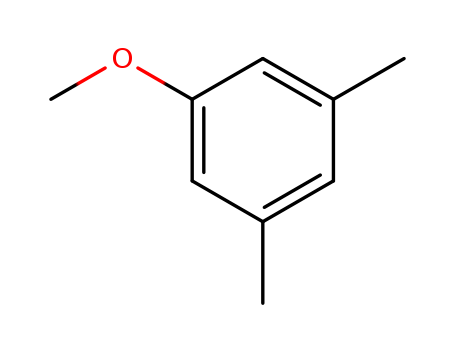
3,5-Dimethylanisole
CAS:874-63-5