64965-48-6
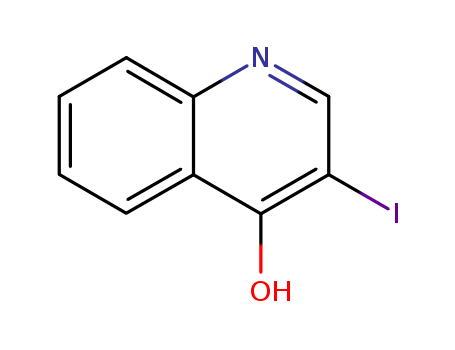
- Product Name:3-IODO-QUINOLIN-4-OL
- Molecular Formula:C9H6INO
- Purity:99%
- Molecular Weight:271.057
Product Details
Factory supply 3-IODO-QUINOLIN-4-OL 64965-48-6 with sufficient stock and high standard
- Molecular Formula:C9H6INO
- Molecular Weight:271.057
- PSA:32.86000
- LogP:2.13270
3-IODO-QUINOLIN-4-OL(Cas 64965-48-6) Usage
|
General Description |
3-Iodo-quinolin-4-ol, also known as 4-hydroxy-3-iodoquinoline, is a chemical compound classified as a part of the quinolines. As suggested by its name, it is a derivative of quinoline with an iodine atom attached to the third carbon atom and a hydroxyl group attached to the fourth carbon atom. This chemical compound is recognized for its broad scope of pharmaceutical applications due to its antimalarial, antibacterial, antifungal, and anticancer properties. However, comprehensive information regarding its safety measures, environmental impact, physicochemical properties, and detailed applications are limited and requires further research for complete characterization. |
InChI:InChI=1S/C9H6INO/c10-7-5-11-8-4-2-1-3-6(8)9(7)12/h1-5H,(H,11,12)
64965-48-6 Relevant articles
AZABICYCLIC(THIO)AMIDES AS FUNGICIDAL COMPOUNDS
-
Page/Page column 142, (2021/11/26)
The present invention relates to azabicy...
TOLL-LIKE RECEPTOR 8 AGONISTS
-
Page/Page column 20; 32; 24; 42, (2015/07/07)
Compounds described herein can be used f...
Exquisite selectivity for human toll-like receptor 8 in substituted furo[2,3-c]quinolines
Kokatla, Hari Prasad,Sil, Diptesh,Malladi, Subbalakshmi S.,Balakrishna, Rajalakshmi,Hermanson, Alec R.,Fox, Lauren M.,Wang, Xinkun,Dixit, Anshuman,David, Sunil A.
, p. 6871 - 6885 (2013/10/01)
Toll-like receptor (TLR)-8 agonists acti...
A simple method for iodination of heterocyclic compounds using HIO 4/NaCl/silica gel/H2SO4 in water
Hosseini, Abolfazl,Khalilzadeh, Mohammad A.,Hosseinzadeh, Masoumeh,Tajbakhsh, Mahmoud
experimental part, p. 619 - 623 (2012/07/14)
A fast and simple method for iodination ...
64965-48-6 Process route
-
-
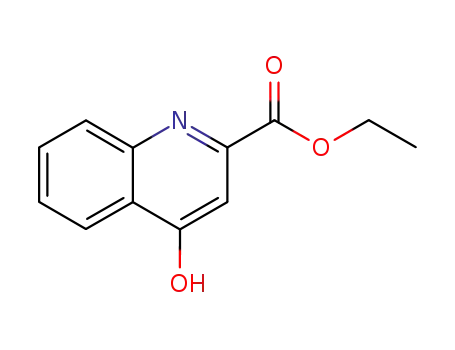
24782-43-2
ethyl 4-hydroxyquinoline-2-carboxylate

-
-
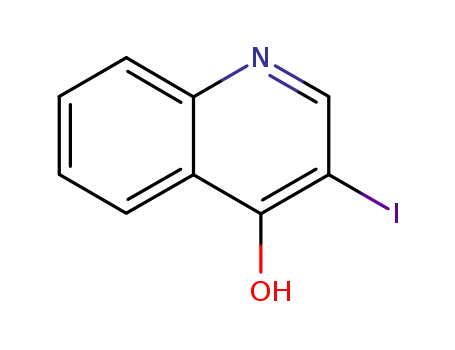
64965-48-6
3-iodoquinolin-4-ol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: acetic acid; ICl / 80 °C
2: aqueous NaOH
3: diphenyl ether; biphenyl / 180 °C
With
sodium hydroxide; diphenylether; biphenyl; Iodine monochloride; acetic acid;
|
-
-
611-36-9
quinolin-4-ol

-

-
64965-48-6
3-iodoquinolin-4-ol
| Conditions | Yield |
|---|---|
|
With
N-iodo-succinimide;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 2h;
|
80% |
|
With
N-iodo-succinimide;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 2h;
|
80% |
|
With
sulfuric acid; silica gel; periodic acid; sodium chloride;
In
water;
at 50 ℃;
for 0.416667h;
|
67% |
|
With
Iodine monochloride; acetic acid;
|
|
|
With
iodine; potassium iodide; sodium hydroxide;
In
water;
at 20 ℃;
for 3h;
|
|
|
With
iodine; potassium iodide; sodium hydroxide;
In
water;
at 20 ℃;
for 3h;
|
1.6 g |
64965-48-6 Upstream products
-
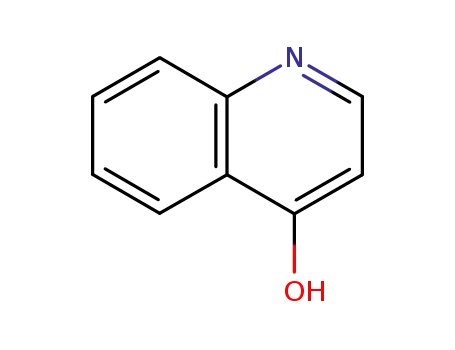
611-36-9

quinolin-4-ol
-
855634-00-3
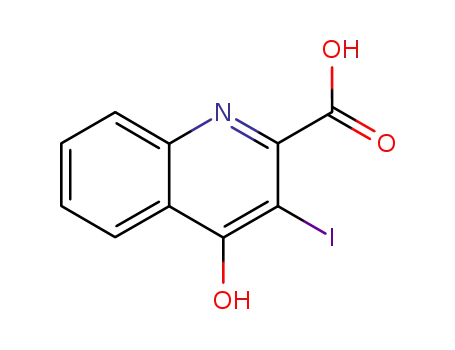
4-hydroxy-3-iodo-quinoline-2-carboxylic acid
-
24782-43-2

ethyl 4-hydroxyquinoline-2-carboxylate
-
855633-99-7
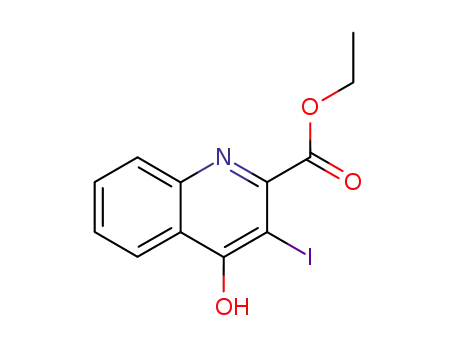
4-hydroxy-3-iodo-quinoline-2-carboxylic acid ethyl ester
64965-48-6 Downstream products
-
1268468-60-5
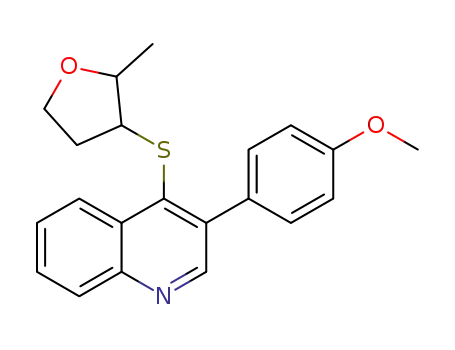
3-(4-methoxy-phenyl)-4-(2-methyl-tetrahydro-furan-3-ylsulfanyl)-quinoline
-
1449680-37-8
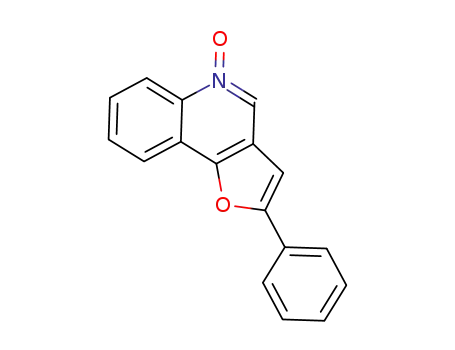
2-phenylfuro[3,2-c]quinoline 5-oxide
-
1449680-40-3
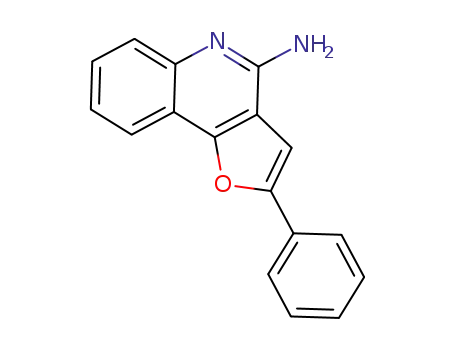
2-phenylfuro[3,2-c]quinolin-4-amine
-
1449680-34-5
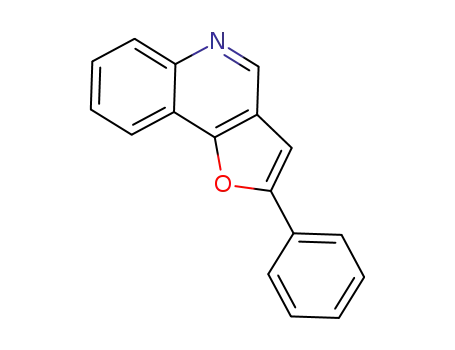
2-phenylfuro[3,2-c]quinoline
Relevant Products
-
4-Chloro-2-iodoaniline
CAS:63069-48-7
-
2-(Perfluorohexyl)ethyl methacrylate
CAS:2144-53-8
-
N-Isopropylacrylamide
CAS:2210-25-5