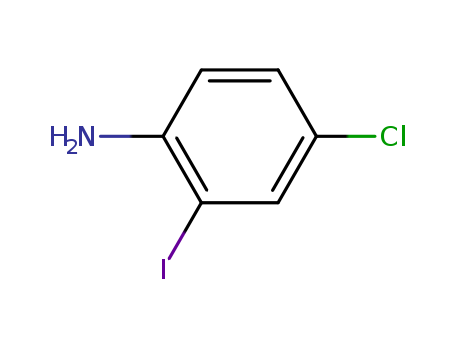
63069-48-7
- Product Name:4-Chloro-2-iodoaniline
- Molecular Formula:C6H5ClIN
- Purity:99%
- Molecular Weight:253.47
Product Details
Chemical plants supply high-quality 4-Chloro-2-iodoaniline 63069-48-7 in bulk
- Molecular Formula:C6H5ClIN
- Molecular Weight:253.47
- Appearance/Colour:amber solid
- Melting Point:40 °C
- Boiling Point:295 °C at 760 mmHg
- PKA:1.90±0.10(Predicted)
- Flash Point:132.2 °C
- PSA:26.02000
- Density:2.015 g/cm3
- LogP:3.10800
63069-48-7 Relevant articles
Au(I)-Catalyzed 6-endo-dig Cyclizations of Aromatic 1,5-Enynes to 2-(Naphthalen-2-yl)anilines Leading to Divergent Syntheses of Benzo[α]carbazole, Benzo[c,h]cinnoline and Dibenzo[i]phenanthridine Derivatives
Fu, Jiayue,Li, Bingbing,Wang, Xiugui,Liang, Qida,Peng, Xiaoshi,Yang, Lu,Wan, Tao,Wang, Xinxiu,Lin, Bin,Cheng, Maosheng,Liu, Yongxiang
supporting information, p. 46 - 52 (2021/11/20)
A gold(I)-catalyzed 6-endo-dig cyclizati...
NCBSI/KI: A Reagent System for Iodination of Aromatics through in Situ Generation of I-Cl
Palav, Amey,Misal, Balu,Chaturbhuj, Ganesh
, p. 12467 - 12474 (2021/08/24)
In situ iodine monochloride (I-Cl) gener...
Sulfated polyborate-H2O assisted tunable activation of N-iodosuccinimide for expeditious mono and diiodination of arenes
Misal, Balu,Palav, Amey,Ganwir, Prerna,Chaturbhuj, Ganesh
supporting information, (2021/05/26)
Owing to both Lewis and Bronsted acid ac...
Cascade Reactions Assisted by Microwave Irradiation: Ultrafast Construction of 2-Quinolinone-Fused γ-Lactones fromN-(o-Ethynylaryl)acrylamides and Formamide
Sacchelli, Bruce A. L.,Rocha, Bianca C.,Andrade, Leandro H.
supporting information, p. 5071 - 5075 (2021/07/20)
An ultrafast (10 s) methodology to const...
63069-48-7 Process route
-
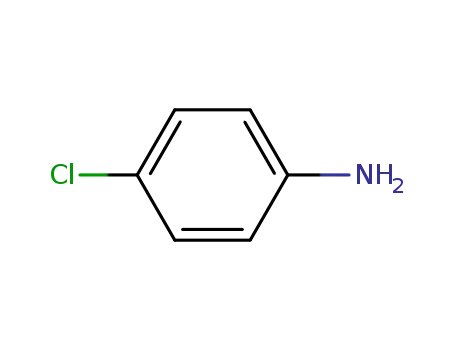
-
106-47-8
4-chloro-aniline

-
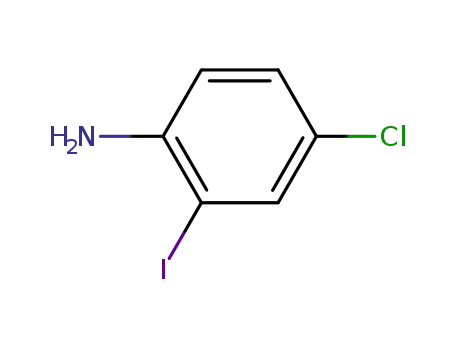
-
63069-48-7
p-chloro-o-iodoaniline

-
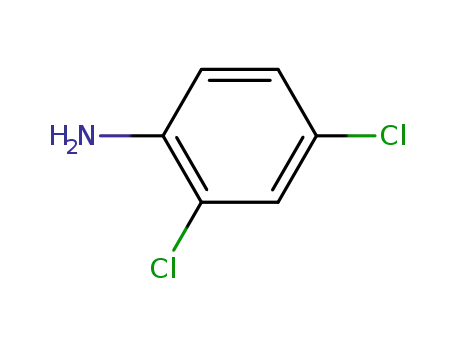
-
554-00-7,51908-09-9
2,4-Dichloroaniline
| Conditions | Yield |
|---|---|
|
With
1-butyl-3-methyl-pyridinium dichloroiodate;
for 1h;
|
79 %Chromat. 73 %Chromat. |
-
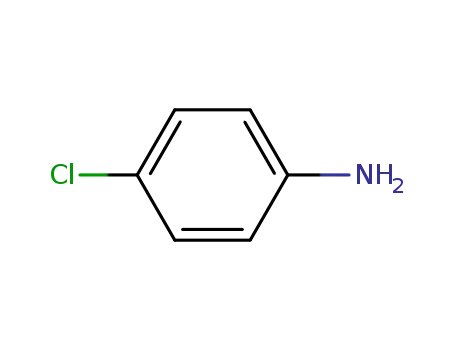
-
106-47-8
4-chloro-aniline

-

-
63069-48-7
p-chloro-o-iodoaniline

-
-
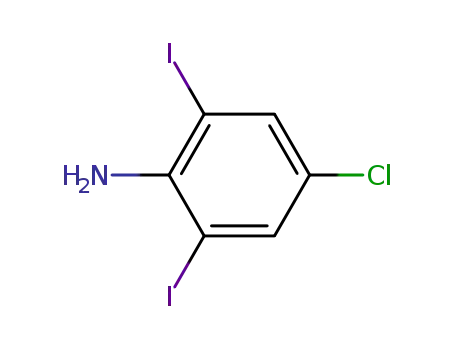
88149-53-5
4-chloro-2,6-diiodoaniline
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; sodium iodine dichloride;
In
methanol; water;
at 20 ℃;
for 3h;
pH=1 - 2;
|
75% 24% |
|
With
hydrogenchloride; potassium iodate; potassium iodide;
In
methanol; water;
at 20 ℃;
for 3h;
|
63069-48-7 Upstream products
-
106-47-8
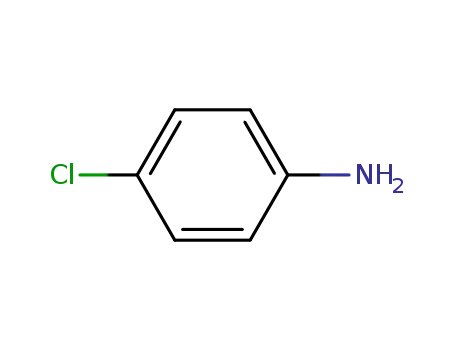
4-chloro-aniline
-
784183-51-3
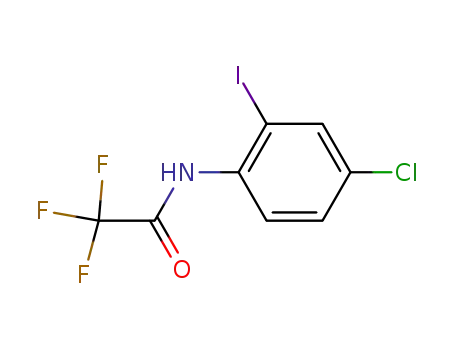
N-(4-chloro-2-iodophenyl)-2,2,2-trifluoroacetamide
-
116035-66-6
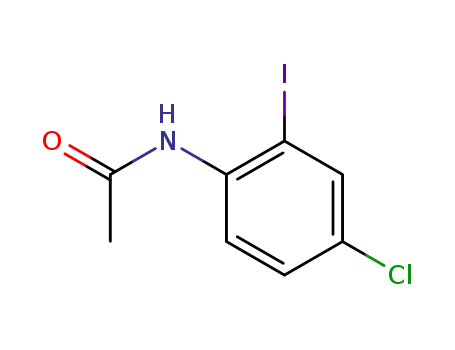
N-(4-chloro-2-iodophenyl)acetamide
-
98-86-2
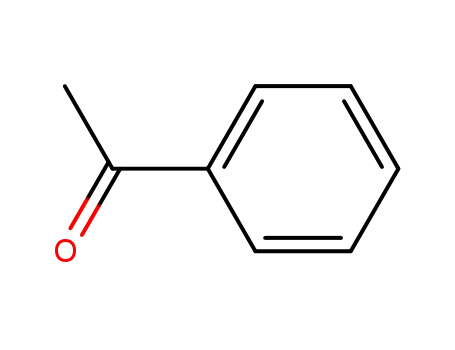
acetophenone
63069-48-7 Downstream products
-
29682-41-5
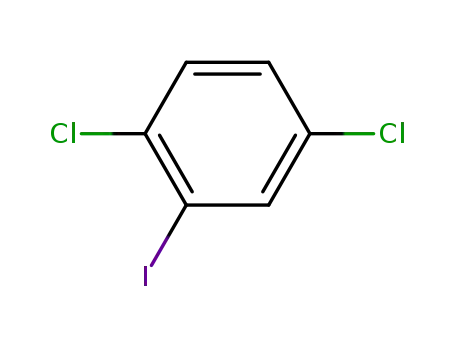
2,5-dichloroiodobenzene
-
88149-53-5

4-chloro-2,6-diiodoaniline
-
116035-66-6

N-(4-chloro-2-iodophenyl)acetamide
-
62835-70-5
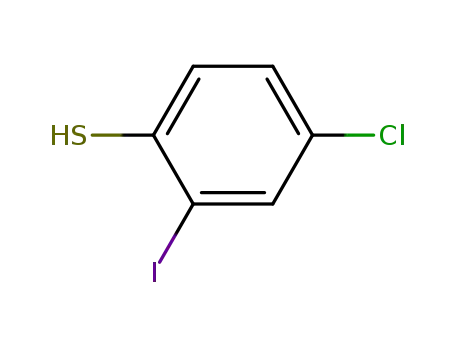
4-chloro-2-iodothiophenol
Relevant Products
-
4-Chloro-o-phenylenediamine
CAS:95-83-0
-
2-HYDROXYACETAMIDE
CAS:598-42-5
-
2-bromo-4-chloropropiophenone
CAS:877-37-2