95-83-0
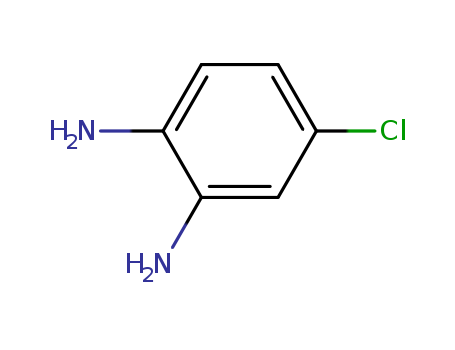
- Product Name:4-Chloro-o-phenylenediamine
- Molecular Formula:C6H7ClN2
- Purity:99%
- Molecular Weight:142.588
Product Details
Factory supply 4-Chloro-o-phenylenediamine 95-83-0 with sufficient production capacity
- Molecular Formula:C6H7ClN2
- Molecular Weight:142.588
- Appearance/Colour:brown crystalline solid or powder
- Vapor Pressure:0.00114mmHg at 25°C
- Melting Point:70-75 °C
- Refractive Index:1.67
- Boiling Point:300.2 °C at 760 mmHg
- PKA:3.52±0.10(Predicted)
- Flash Point:135.4 °C
- PSA:52.04000
- Density:1.345 g/cm3
- LogP:2.66680
4-Chloro-1,2-diaminobenzene(Cas 95-83-0) Usage
|
Production Methods |
Commercial production of 4-chloro-o-PDA in the United States was first reported in 1941. About 0.45–4.5 thousand kg was produced by a U.S. company in 1977. It was also produced by one company in the Federal Republic of Germany. This compound has been patented as a hair dye component, fur dyes, inks, and hair coloring formulations, and is believed to be used to produce 5-chlorobenzotriazole, an isomer of which is a photographic chemical. The primary routes of potential human exposure to 4- chloro-o-PDA are inhalation and dermal contact during its production. Consumer exposure may occur from use of hair dyes or products made from 5-chlorobenzotriazole. |
|
Air & Water Reactions |
4-Chloro-1,2-diaminobenzene may be sensitive to prolonged exposure to air and light. Insoluble in water. |
|
Reactivity Profile |
4-Chloro-1,2-diaminobenzene is incompatible with strong oxidizers. 4-Chloro-1,2-diaminobenzene reacts with alpha-ketoacids to form quinoxalones. |
|
Fire Hazard |
Flash point data for 4-Chloro-1,2-diaminobenzene are not available. 4-Chloro-1,2-diaminobenzene is probably combustible. |
|
Safety Profile |
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Human mutation data reported. When heated to decomposition it emits toxic fumes of Cland NOx. See also AROMATIC AMINES. |
|
Potential Exposure |
This organochlorine material has been patented as a hair dye component. It is believed to be used in production of photographic chemicals. In varying degrees, organochlorines are absorbed from the gut and also by the lung and across the skin. |
|
Carcinogenicity |
4-Chloro-o-phenylenediamine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity fromstudies in experimental animals. |
|
Purification Methods |
Recrystallise the diamine from pet. ether. [Beilstein 13 IV 68.] |
|
Incompatibilities |
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Light sensitive. It reacts with alpha-ketoacids to form quinoxalones or benzopyrazines. |
|
Definition |
ChEBI: 4-Chloro-ortho-phenylenediamine is a member of monochlorobenzenes. Undergoes cyclizations and cyclocondensations to form benzimidazoles. |
|
General Description |
4-chloro-o-phenylenediamine appears as brown crystalline solid or powder. (NTP, 1992) |
InChI:InChI=1/C6H7ClN2/c7-4-1-2-5(8)6(9)3-4/h1-3H,8-9H2
95-83-0 Relevant articles
-
Maki,Hashimoto
, p. 602,603 (1954)
-
Scalable continuous flow hydrogenations using Pd/Al2O3-coated rectangular cross-section 3D-printed static mixers
Lebl, René,Zhu, Yutong,Ng, Derrick,Hornung, Christian H.,Cantillo, David,Kappe, C. Oliver
, p. 55 - 63 (2022)
A novel type of catalytic static mixers ...
Efficient and recyclable bimetallic Co–Cu catalysts for selective hydrogenation of halogenated nitroarenes
Lu, Xionggang,Ren, Jiaan,Sheng, Yao,Wang, Xueguang,Wu, Baoqin,Zou, Xiujing
, (2021/12/20)
Silica supported N-doped carbon layers e...
ARYL HYDROCARBON RECEPTOR ACTIVATORS
-
Page/Page column 46; 50; 51-53, (2021/02/05)
Small molecule AhR ligands are disclosed...
Aluminum Metal-Organic Framework-Ligated Single-Site Nickel(II)-Hydride for Heterogeneous Chemoselective Catalysis
Antil, Neha,Kumar, Ajay,Akhtar, Naved,Newar, Rajashree,Begum, Wahida,Dwivedi, Ashutosh,Manna, Kuntal
, p. 3943 - 3957 (2021/04/12)
The development of chemoselective and he...
95-83-0 Process route
-
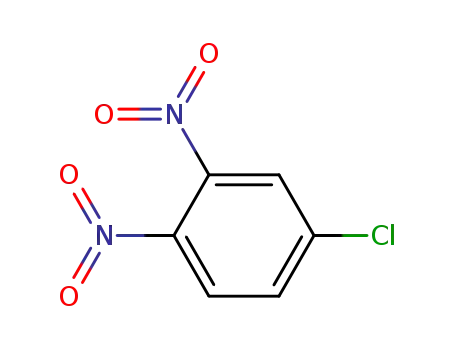
-
610-40-2
3,4-dinitro-chlorobenzene

-
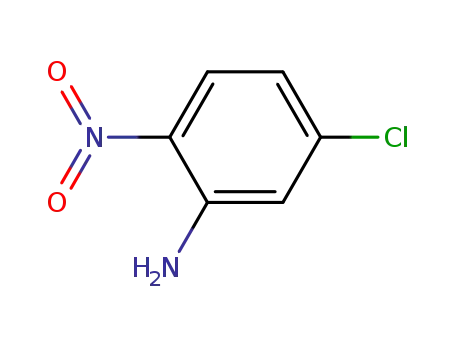
-
1635-61-6
5-chloro-2-nitroaniline

-
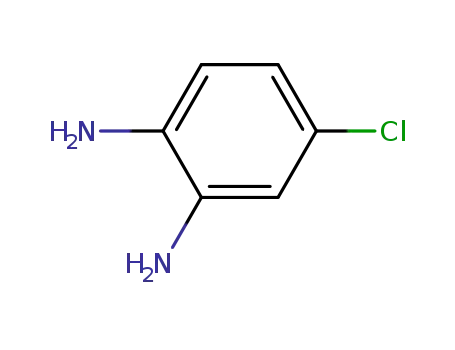
-
95-83-0
4-Chloro-1,2-phenylenediamine

-
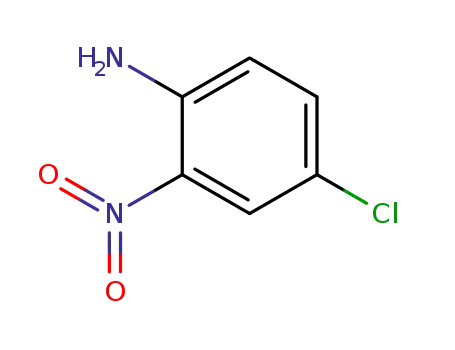
-
89-63-4
4-Chloro-2-nitroaniline
| Conditions | Yield |
|---|---|
|
With
tin(ll) chloride;
In
methanol; water;
Title compound not separated from byproducts;
|
23.66 % Chromat. 11.03 % Chromat. 12.20 % Chromat. |
-
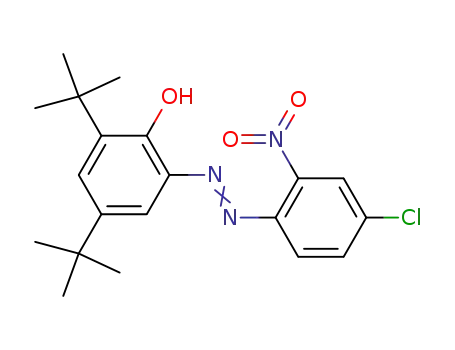
-
52184-29-9
2-nitro-4-chloro-2'-hydroxy-3',5'-di-tert-butylazobenzene

-

-
95-83-0
4-Chloro-1,2-phenylenediamine

-
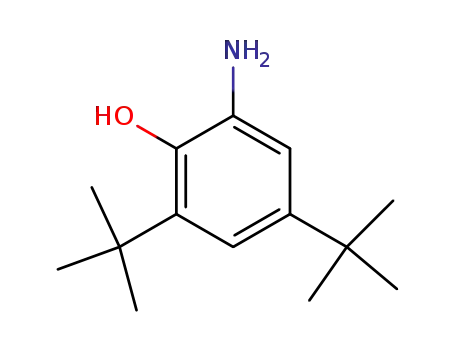
-
1643-39-6
2-amino-4,6-di-tertbutylphenol

-
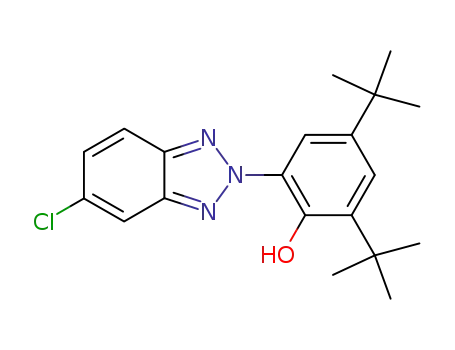
-
3864-99-1
2-(5'-chloro-2'H-benzotriazol-2'-yl)-4,6-di-tert-butylphenol
| Conditions | Yield |
|---|---|
|
With
2-bromo-2-nitropropane; zinc;
In
methanol; dichloromethane;
for 4h;
Ambient temperature;
|
88 % Chromat. |
95-83-0 Upstream products
-
89-63-4

4-Chloro-2-nitroaniline
-
610-40-2

3,4-dinitro-chlorobenzene
-
1635-61-6

5-chloro-2-nitroaniline
-
17348-69-5
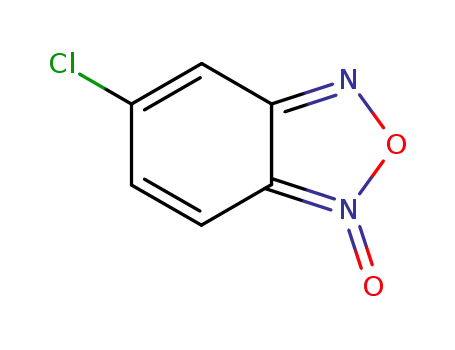
5-chlorobenzofurazan-1-oxide
95-83-0 Downstream products
-
82138-56-5
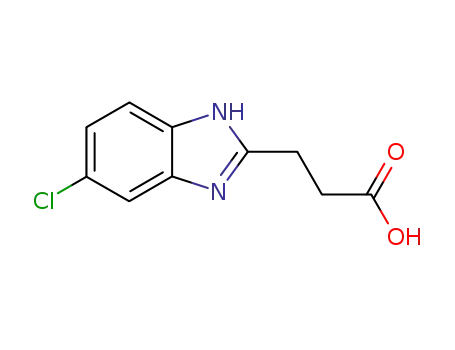
3-(5-chloro-1H-benzo[d]imidazol-2-yl)propanoic acid
-
109261-24-7
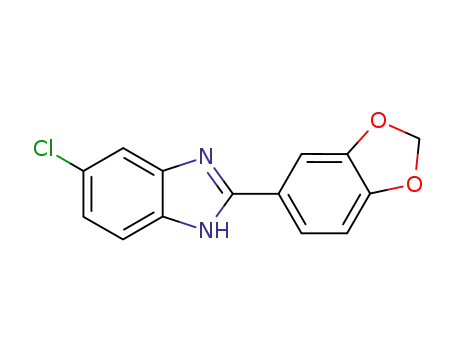
2-benzo[1,3]dioxol-5-yl-5-chloro-1H-benzimidazole
-
116636-82-9
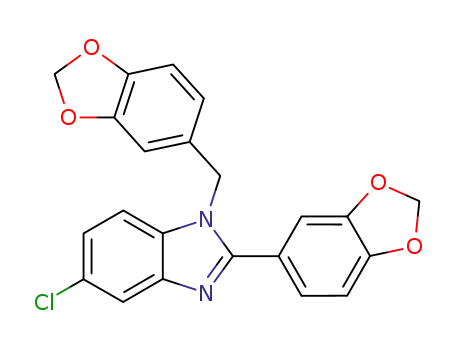
2-benzo[1,3]dioxol-5-yl-5-chloro-1-piperonyl-1H-benzimidazole
-
2047-60-1
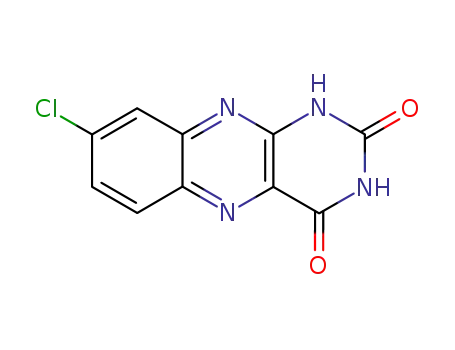
8-chloro-1H-benzo[g]pteridine-2,4-dione
Relevant Products
-
4-Aminobenzamidine dihydrochloride
CAS:2498-50-2
-
GLYCERYL PROPOXY TRIACRYLATE
CAS:52408-84-1
-
4,5-epoxytetrahydrophthalic acid diglycidylester
CAS:25293-64-5