2210-25-5
- Product Name:N-Isopropylacrylamide
- Molecular Formula:C6H11NO
- Purity:99%
- Molecular Weight:113.159
Product Details
China cas 2210-25-5 factory wholesale N-Isopropylacrylamide at affordable price
- Molecular Formula:C6H11NO
- Molecular Weight:113.159
- Appearance/Colour:white to light yellow crystalline powder
- Vapor Pressure:0.076mmHg at 25°C
- Melting Point:60-63 °C(lit.)
- Refractive Index:1.445
- Boiling Point:225.074 °C at 760 mmHg
- PKA:14.83±0.46(Predicted)
- Flash Point:119.664 °C
- PSA:29.10000
- Density:0.877 g/cm3
- LogP:1.08790
N-Isopropylacrylamide(Cas 2210-25-5) Usage
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Fractionate the amide under reduced pressure, and recrystallise the solid distillate from hexane (m 59o), *C6H6 (m 62o) or *C6H6/hexane (m 62-63o). Store it with 0.05% of 4-tert-butylcatechol. It is used for making water soluble swellable hydrogels. [Beilstein 4 IV 517.] |
|
Application |
N-Isopropylacrylamide (NIPAM) can be used to prepare poly(NIPAM) based thermosetting polymers, which can be used for a variety of applications such as tissue engineering, cell culture, biomedical coating, drug delivery, and muscle regeneration.Monomer used in the preparation of thermally sensitive, water-swellable hydrogels. |
|
General Description |
N-Isopropylacrylamide (NIPAM) is a biocompatible monomeric unit that can be used in the formation of stimuli responsive polymers due to its temperature sensitive properties. These properties include temperature based volumetric and phase changes, which change when the temperature of the solution reaches a lower critical solution temperature (LCST). |
InChI:InChI=1/C6H11NO/c1-4(2)5(3)6(7)8/h4H,3H2,1-2H3,(H2,7,8)
2210-25-5 Relevant articles
A ritter-type reaction over H-ZSM-5: Synthesis of N-isopropylacrylamide from acrylonitrile and isopropyl alcohol
Chen, Xin,Okuhara, Toshio
, p. 194 - 201 (2002)
Catalytic synthesis of N-isopropylacryla...
Iridium-Catalyzed Asymmetric Hydroalkenylation of Norbornene Derivatives
Sun, Xin,Bai, Xiao-Yan,Li, An-Zhen,Li, Bi-Jie
supporting information, p. 2182 - 2187 (2021/03/01)
Transition-metal-catalyzed asymmetric hy...
Reactivity of secondary N-alkyl acrylamides in Morita–Baylis–Hillman reactions
Ahmar, Mohammed,Queneau, Yves,Verrier, Charlie,Yue, Xiaoyang
, p. 319 - 330 (2021/10/29)
The Morita–Baylis–Hillman (MBH) reaction...
Method for synthesizing acrylamide derivative
-
Paragraph 0029; 0052; 0056, (2019/10/01)
The invention relates to a method for sy...
Synthesis process of N-isopropylacrylamide
-
Paragraph 0023, (2018/04/01)
The invention relates to a synthesis pro...
2210-25-5 Process route
-
-
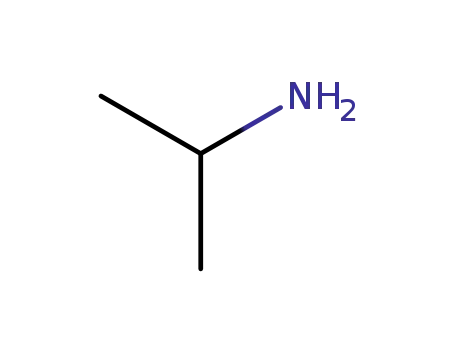
75-31-0
isopropylamine

-
-
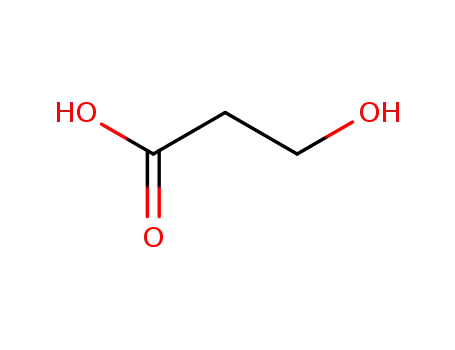
503-66-2
3-hydroxypropionic acid

-
-
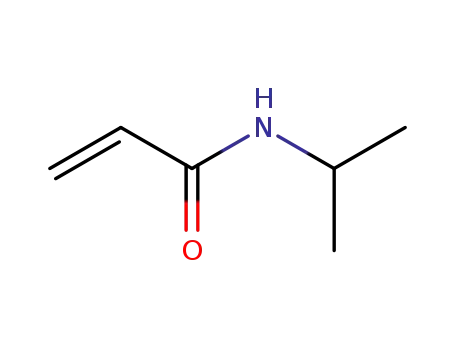
2210-25-5
N-Isopropylacrylamide

-
-
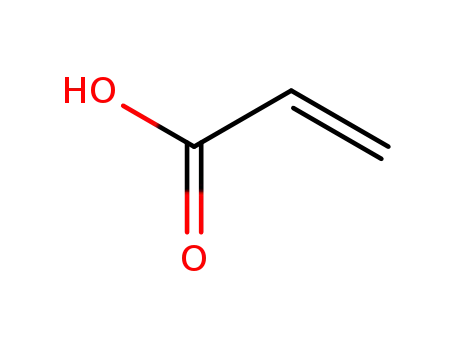
79-10-7
acrylic acid
| Conditions | Yield |
|---|---|
|
silica gel;
at 250 ℃;
Gas phase;
|
24.1% 36.4% |
-
-
C9H19NO2

-

-
2210-25-5
N-Isopropylacrylamide
| Conditions | Yield |
|---|---|
|
With
4-methoxy-phenol; lithium tert-butoxide;
In
glycerol;
at 230 ℃;
Pyrolysis;
|
90% |
2210-25-5 Upstream products
-
75-31-0

isopropylamine
-
292638-85-8
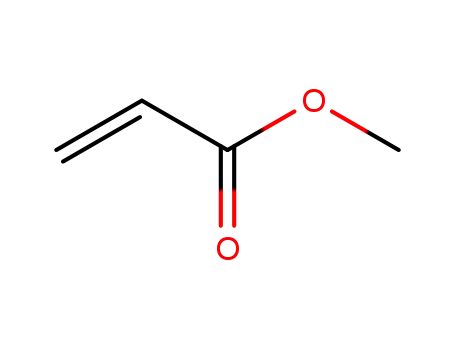
acrylic acid methyl ester
-
79-10-7

acrylic acid
-
22813-48-5
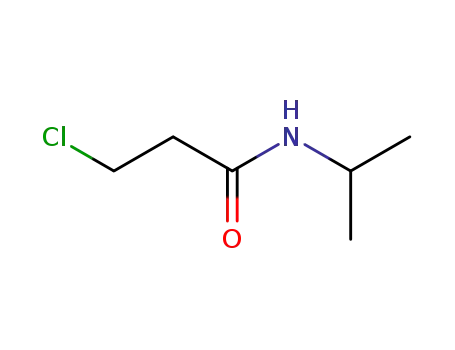
3-chloro-propionic acid isopropylamide
2210-25-5 Downstream products
-
57181-29-0
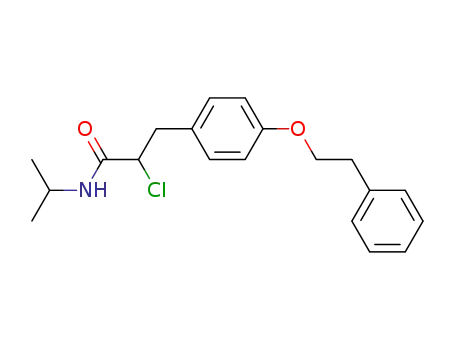
2-Chloro-N-isopropyl-3-(4-phenethyloxy-phenyl)-propionamide
-
23784-47-6
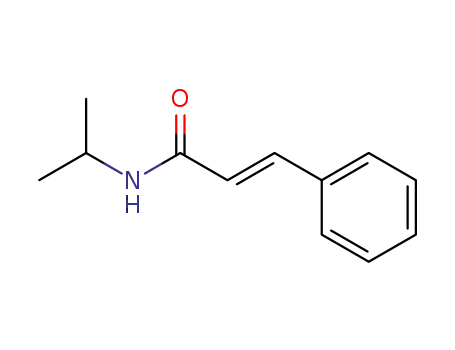
(2E)-N-isopropyl-3-phenylacrylamide
-
56146-87-3

N-isopropyl-3-phenylpropanamidate
Relevant Products
-
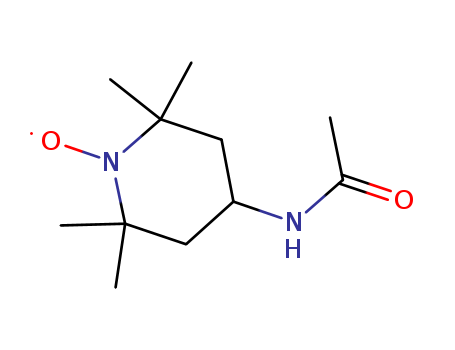
4-ACETAMIDO-TEMPO
CAS:14691-89-5
-
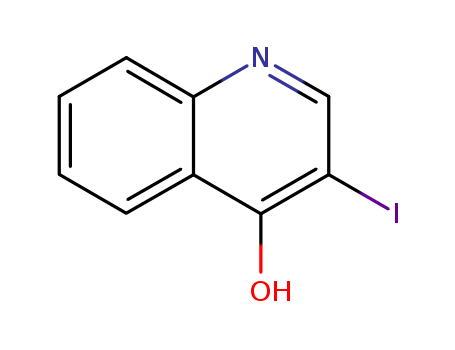
3-IODO-QUINOLIN-4-OL
CAS:64965-48-6
-
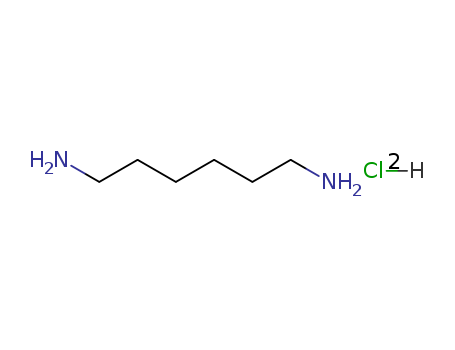
1,6-HEXANEDIAMINE DIHYDROCHLORIDE
CAS:6055-52-3