104-82-5
- Product Name:4-Methylbenzyl chloride
- Molecular Formula:C8H9Cl
- Purity:99%
- Molecular Weight:140.612
Product Details
Purity 99% Min 4-Methylbenzyl chloride 104-82-5 Spot Supply with Safe Transportation
- Molecular Formula:C8H9Cl
- Molecular Weight:140.612
- Appearance/Colour:clear colourless to light yellow liquid
- Vapor Pressure:0.504mmHg at 25°C
- Melting Point:4 °C(lit.)
- Refractive Index:n20/D 1.533(lit.)
- Boiling Point:198.6 °C at 760 mmHg
- Flash Point:75.6 °C
- PSA:0.00000
- Density:1.054 g/cm3
- LogP:2.73380
4-Methylbenzyl chloride(Cas 104-82-5) Usage
|
Purification Methods |
Dry the chloride with CaSO4 and fractionally distil it under vacuum. [Beilstein 5 H 384, 5 III 854, 5 IV 966.] |
InChI:InChI=1/C8H9Cl/c1-7-2-4-8(6-9)5-3-7/h2-5H,6H2,1H3
104-82-5 Relevant articles
A novel synthesis of tolunitriles by selective ammoxidation
Xie, Guangyong,Zhang, Aiqing,Huang, Chi
, p. 969 - 973 (2010)
A new approach to synthesize tolunitrile...
-
Rueggeberg,Ginsburg,Frantz
, p. 2154 (1945)
-
-
Dalrymple et al.
, p. 2647 (1964)
-
-
Shacklett,Smith
, p. 766 (1951)
-
-
Shiner et al.
, p. 4838,4840 (1969)
-
Microwave assisted solid additive effects in simple dry chlorination reactions with n-chlorosuccinimide
Bucos, Madalina,Villalonga-Barber, Carolina,Micha-Screttas, Maria,Steele, Barry R.,Screttas, Constantinos G.,Heropoulos, Georgios A.
, p. 2061 - 2065 (2010)
Solid additives participate in the dry m...
Regiospecific chlorination of xylenes using K-10 montmorrillonite clay
Thirumamagal,Narayanasamy, Sureshbabu,Venkatesan
, p. 2820 - 2825 (2008)
Regiospecific chlorination of xylenes ha...
Facile synthesis of hypercrosslinked resin via photochlorination of p-xylene and succedent alkylation polymerization
Bai, Ling Ling,Zhou, Yong Hao,Wang, Xiu Li,Yuan, Si Guo,Wu, Xian Li
, p. 1115 - 1118 (2011)
A combination of photochlorination of p-...
Photocatalytic activation of N-chloro compounds for the chlorination of arenes
Hering, Thea,K?nig, Burkhard
, p. 7821 - 7825 (2016)
Photoredox catalysis activates N-chloram...
Efficient synthesis of tolunitriles by selective ammoxidation
Xie, Guangyong,Zheng, Qiong,Huang, Chi,Chen, Yuanyin
, p. 1103 - 1107 (2003)
Tolunitriles have been efficiently synth...
Mechanism of solvolysis of substituted benzyl chlorides in aqueous ethanol
Denegri, Bernard,Mati?, Mirela,Va?ko, Monika
supporting information, (2021/11/22)
The mechanism of solvolyses of activated...
Structure-guided optimization of 1H-imidazole-2-carboxylic acid derivatives affording potent VIM-Type metallo-β-lactamase inhibitors
Yan, Yu-Hang,Li, Wenfang,Chen, Wei,Li, Chao,Zhu, Kai-Rong,Deng, Ji,Dai, Qing-Qing,Yang, Ling-Ling,Wang, Zhenling,Li, Guo-Bo
, (2021/11/17)
Production of metallo-β-lactamases (MBLs...
Nickel catalyzed deoxygenative cross-coupling of benzyl alcohols with aryl-bromides
Kumar Chenniappan, Vinoth,Peck, Devin,Rahaim, Ronald
, (2020/03/03)
A nickel-catalyzed cross-electrophile co...
N -Hydroxyphthalimide/benzoquinone-catalyzed chlorination of hydrocarbon C-H bond using N -chlorosuccinimide
Li, Zi-Hao,Fiser, Béla,Jiang, Biao-Lin,Li, Jian-Wei,Xu, Bao-Hua,Zhang, Suo-Jiang
supporting information, p. 3403 - 3408 (2019/04/01)
The direct chlorination of C-H bonds has...
104-82-5 Process route
-
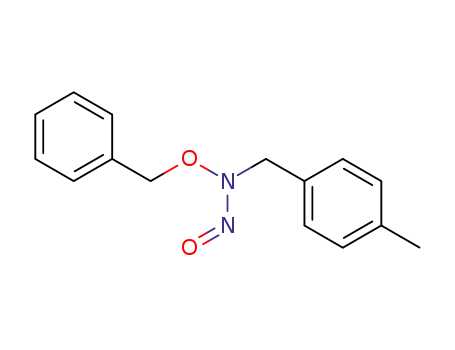
-
146085-66-7
N-(p-methylbenzyl)-O-benzyl-N-nitrosohydroxylamine

-
-
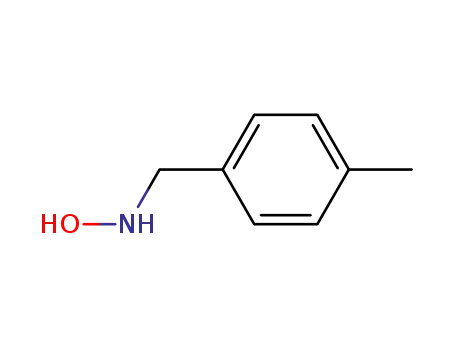
16814-17-8
N-(p-methylbenzyl)hydroxylamine

-
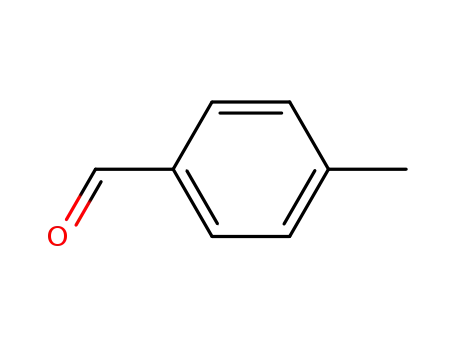
-
104-87-0
4-methyl-benzaldehyde

-
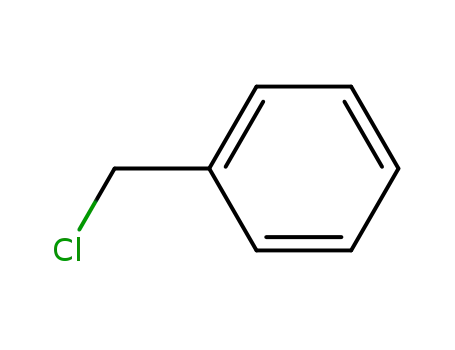
-
100-44-7
benzyl chloride

-

-
104-82-5
4-Methylbenzyl chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
for 5h;
Further byproducts given;
Heating;
|
0.1 mmol 0.7 mmol 3.0 mmol 0.11 g |
-

-
146085-66-7
N-(p-methylbenzyl)-O-benzyl-N-nitrosohydroxylamine

-

-
16814-17-8
N-(p-methylbenzyl)hydroxylamine

-
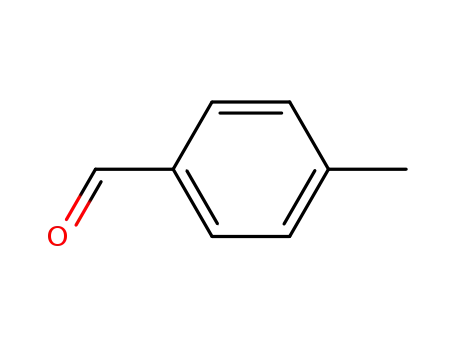
-
104-87-0
4-methyl-benzaldehyde

-
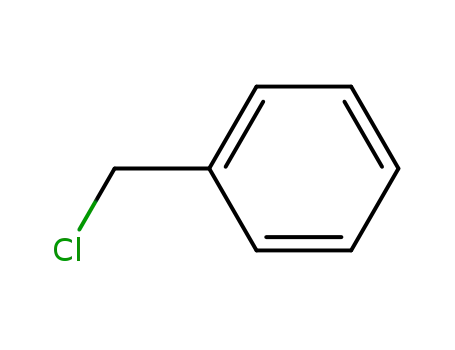
-
100-44-7
benzyl chloride

-

-
104-82-5
4-Methylbenzyl chloride

-
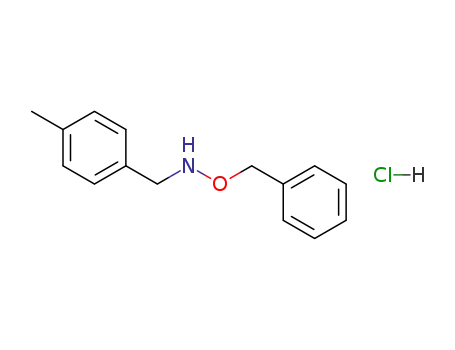
-
146085-60-1
N-(p-methylbenzyl)-O-benzylhydroxylamine hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
at 70 - 90 ℃;
for 5h;
Product distribution;
Mechanism;
other temp.; acid-catalyzed hydrolysis of other N,O-dibenzyl-N-nitrosohydroxylamine;
|
2.0 mmol 0.4 mmol 0.5 mmol 0.2 mmol 0.8 mmol |
104-82-5 Upstream products
-
110-88-3
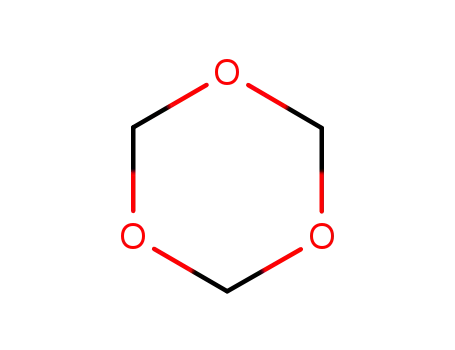
1,3,5-Trioxan
-
108-88-3
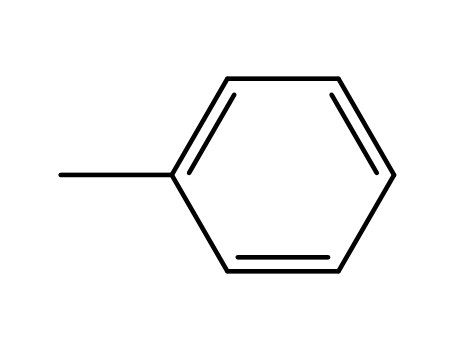
toluene
-
128-09-6
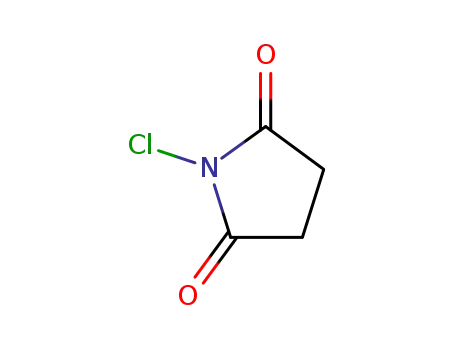
N-chloro-succinimide
-
106-42-3
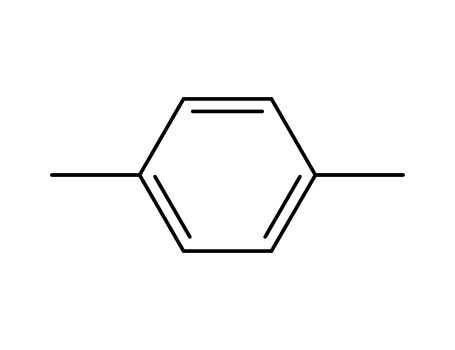
para-xylene
104-82-5 Downstream products
-
104-84-7
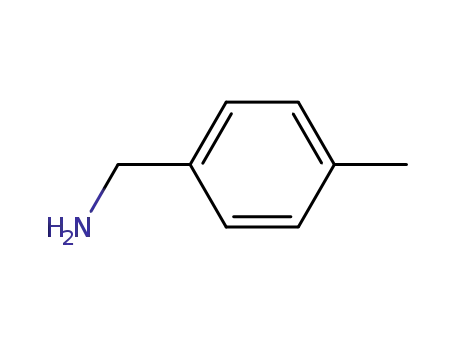
para-methylbenzylamine
-
351-68-8
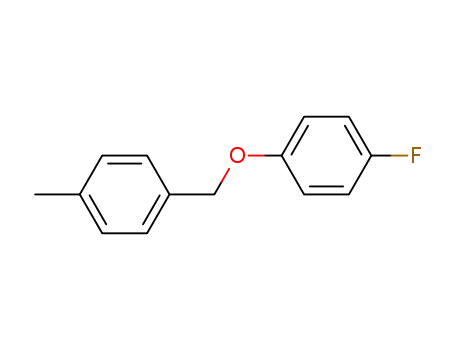
(4-fluoro-phenyl)-(4-methyl-benzyl)-ether
-
713130-52-0
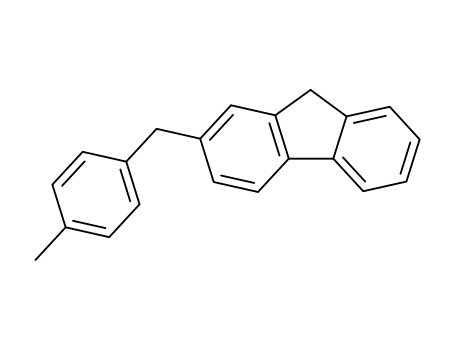
2-(4-methyl-benzyl)-fluorene
-
859776-61-7
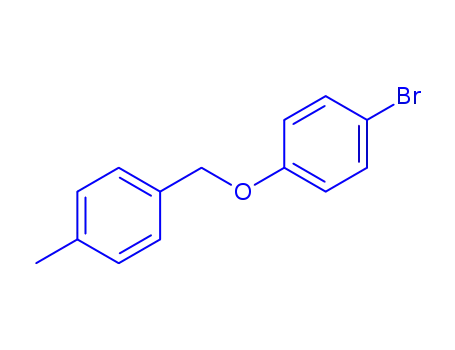
(4-bromo-phenyl)-(4-methyl-benzyl)-ether
Relevant Products
-
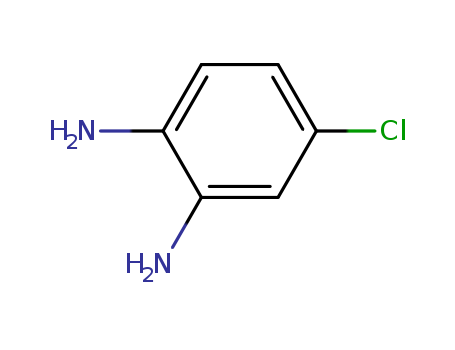
4-Chloro-o-phenylenediamine
CAS:95-83-0
-
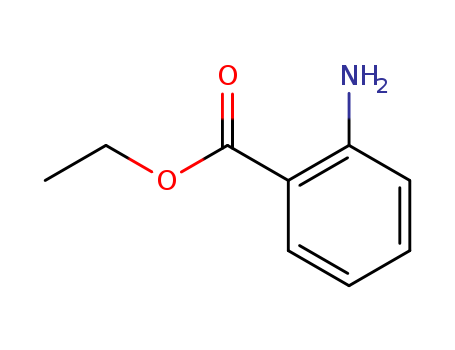
ETHYL ANTHRANILATE
CAS:87-25-2
-
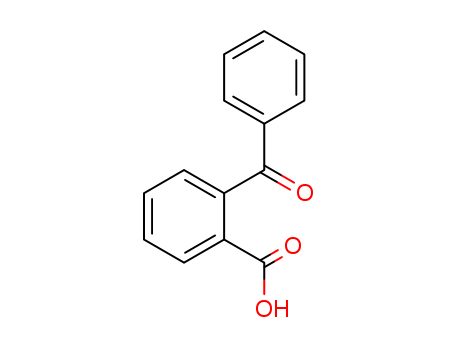
2-Benzoylbenzoic acid
CAS:85-52-9