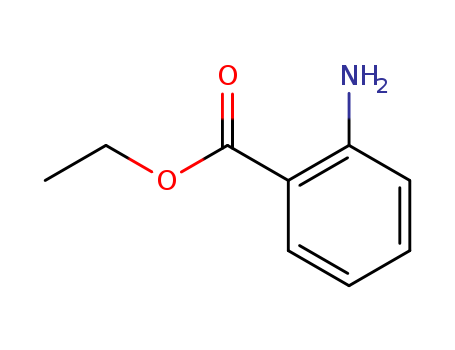
87-25-2
- Product Name:ETHYL ANTHRANILATE
- Molecular Formula:C9H11NO2
- Purity:99%
- Molecular Weight:165.192
Product Details
Reputable factory supply ETHYL ANTHRANILATE 87-25-2 in stock with high standard
- Molecular Formula:C9H11NO2
- Molecular Weight:165.192
- Appearance/Colour:colourless liquid
- Vapor Pressure:0.00954mmHg at 25°C
- Melting Point:13-15 °C(lit.)
- Refractive Index:n20/D 1.564(lit.)
- Boiling Point:264.7 °C at 760 mmHg
- PKA:2.20±0.10(Predicted)
- Flash Point:126.5 °C
- PSA:52.32000
- Density:1.13 g/cm3
- LogP:2.02670
ETHYL ANTHRANILATE(Cas 87-25-2) Usage
|
Preparation |
By esterification of anthranilic acid with ethanol in the presence of acid catalysts; by reacting sodium hypochlorite with an alkaline solution of phthalimide |
|
Air & Water Reactions |
ETHYL ANTHRANILATE should be protected from air and light. Insoluble in water. |
|
Reactivity Profile |
ETHYL ANTHRANILATE may hydrolyze under acidic and basic conditions. |
|
Fire Hazard |
Flash point data for ETHYL ANTHRANILATE are not available. ETHYL ANTHRANILATE is probably combustible. |
|
Pharmacology |
Ethyl anthranilate behaved as a local anaesthetic, as measured by depression of muscle twitch, but was 50% less effective than the m- and p-aminobenzoate isomers. All three compounds potentiated the initial phase of caffeine-induced contracture of frog sartorius muscle, but the o-isomer had no effect on the peak tension of the contracture (Friedman, Bianchi & Weiss. 1974). It was proposed that the o-amino group has both steric and polar effects, its bulk preventing the inhibitory action of the carbonyl group on the caffeine-induced contracture of sartorius muscle, while the lone electron pair of the nitrogen atom induces a contracture on its own accord and potentiates a caffeine-induced contracture (Friedman, 1975). Ethyl anthranilate (0-1 mmol/litre) decreased the frequency of the electric-organ discharges of the electric fish, Gnathonemus moori, but did not affect the individual pulse amplitudes, indicating that the compound acted on the pacemaker cells of the mesencephalic command nucleus (Walsh & Schopp, 1966). |
|
Safety Profile |
Moderately toxic by ingestion. A skin irritant. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx. |
|
General Description |
Colorless liquid with a fruity odor. Insoluble in water. |
InChI:InChI=1/C9H11NO2/c1-2-12-9(11)7-5-3-4-6-8(7)10/h3-6H,2,10H2,1H3
87-25-2 Relevant articles
Nickel Boride Catalyzed Reductions of Nitro Compounds and Azides: Nanocellulose-Supported Catalysts in Tandem Reactions
Proietti, Giampiero,Prathap, Kaniraj Jeya,Ye, Xinchen,Olsson, Richard T.,Dinér, Peter
, p. 133 - 146 (2021/11/04)
Nickel boride catalyst prepared in situ ...
Design, synthesis, in vitro and in vivo evaluation against MRSA and molecular docking studies of novel pleuromutilin derivatives bearing 1, 3, 4-oxadiazole linker
Liu, Jie,Zhang, Guang-Yu,Zhang, Zhe,Li, Bo,Chai, Fei,Wang, Qi,Zhou, Zi-Dan,Xu, Ling-Ling,Wang, Shou-Kai,Jin, Zhen,Tang, You-Zhi
, (2021/05/17)
A class of pleuromutilin derivatives con...
Tunable Electrosynthesis of Anthranilic Acid Derivatives via a C-C Bond Cleavage of Isatins
Qian, Peng,Liu, Jiaojiao,Zhang, Yan,Wang, Zhiyong
, p. 16008 - 16015 (2021/07/31)
A facile and direct electrocatalytic C-C...
Overcoming the Deallylation Problem: Palladium(II)-Catalyzed Chemo-, Regio-, and Stereoselective Allylic Oxidation of Aryl Allyl Ether, Amine, and Amino Acids
Begam, Hasina Mamataj,Jana, Ranjan,Manna, Kartic,Samanta, Krishanu
supporting information, p. 7443 - 7449 (2020/10/09)
We report herein a Pd(II)/bis-sulfoxide-...
87-25-2 Process route
-
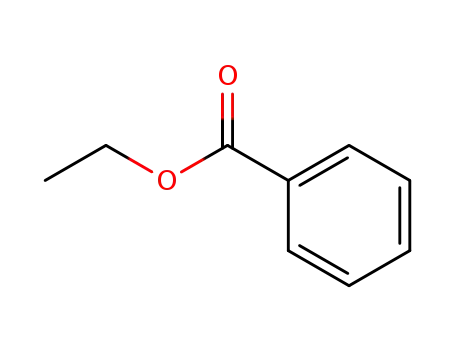
-
93-89-0,99341-95-4
benzoic acid ethyl ester

-
-
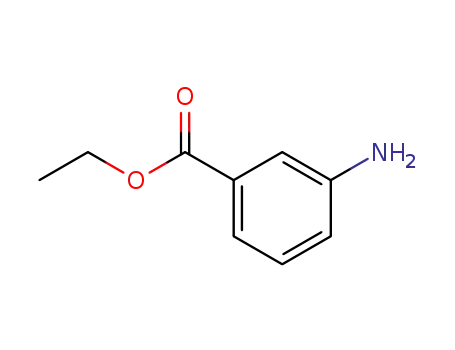
582-33-2
3-aminobenzoic acid ethyl ester

-
-
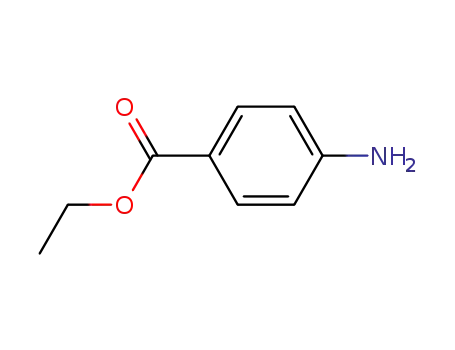
94-09-7
p-aminoethylbenzoate

-
-
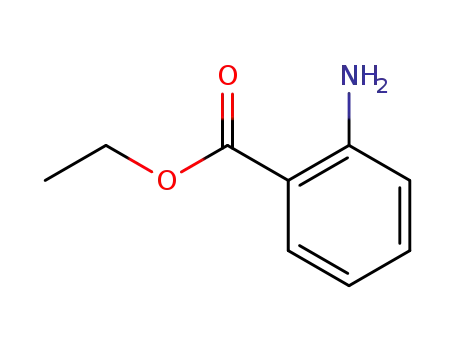
87-25-2
2-ethoxycarbonylaniline
| Conditions | Yield |
|---|---|
|
With
hydroxylamine-O-sulfonic acid;
iron(II) sulfate;
In
water; acetic acid;
at 40 ℃;
for 2h;
Yield given. Yields of byproduct given;
|
|
|
With
hydroxylamine-O-sulfonic acid;
iron(II) sulfate;
In
water; acetic acid;
at 40 ℃;
for 2h;
Product distribution;
Mechanism;
k(C6H5COOEt)/k(C6H6);
|
-
-
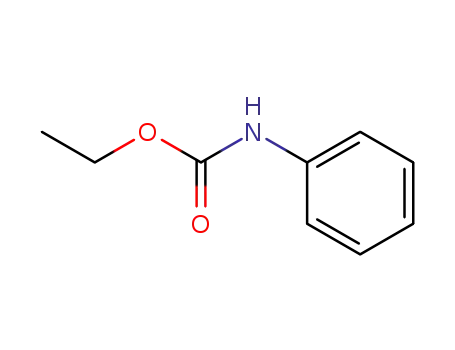
101-99-5
N-carboethoxyaniline

-
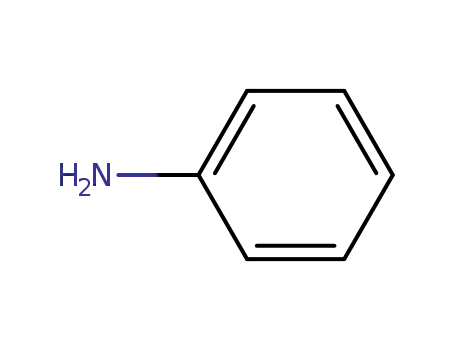
-
62-53-3
aniline

-

-
94-09-7
p-aminoethylbenzoate

-

-
87-25-2
2-ethoxycarbonylaniline
| Conditions | Yield |
|---|---|
|
In
cyclohexane;
Quantum yield;
Mechanism;
Irradiation;
|
87-25-2 Upstream products
-
136918-14-4
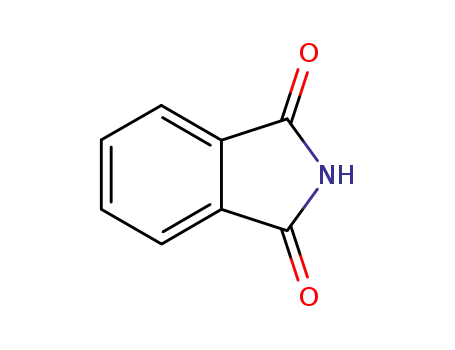
phthalimide
-
118-48-9
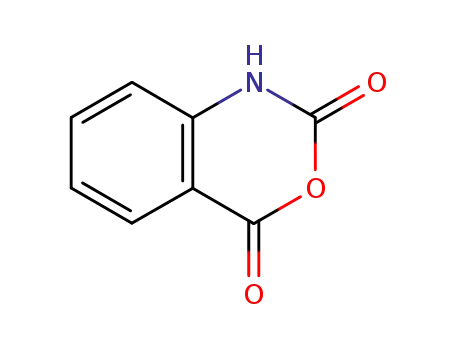
isatoic anhydride
-
64-17-5
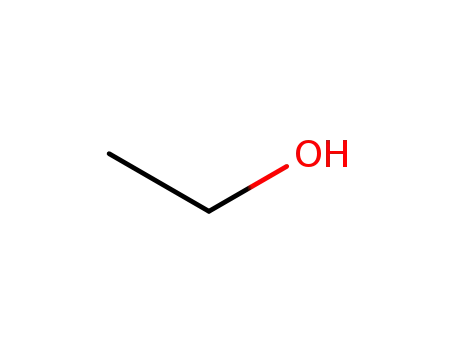
ethanol
-
793634-65-8
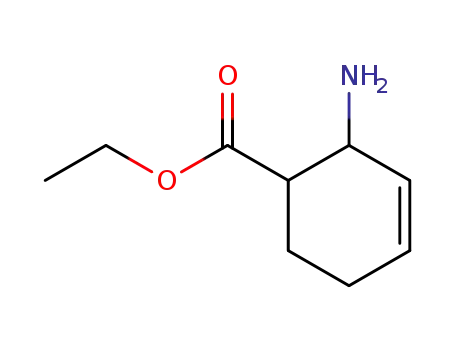
2-amino-cyclohex-3-enecarboxylic acid ethyl ester
87-25-2 Downstream products
-
857486-15-8
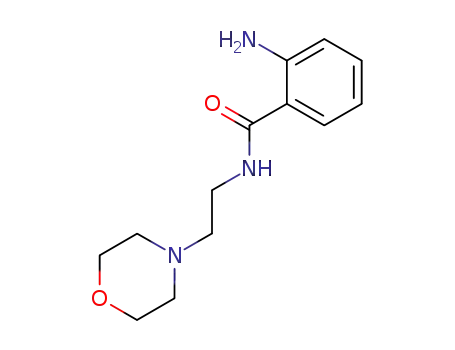
2-amino-N-(2-morpholin-4-yl-ethyl)-benzamide
-
108717-33-5
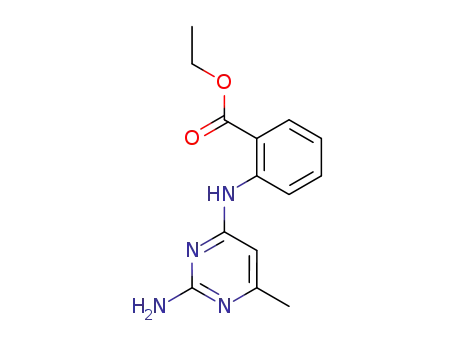
N-(2-amino-6-methyl-pyrimidin-4-yl)-anthranilic acid ethyl ester
-
108718-86-1
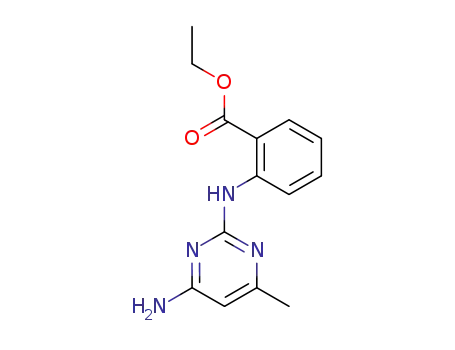
N-(4-amino-6-methyl-pyrimidin-2-yl)-anthranilic acid ethyl ester
-
5335-99-9
2-{[(8-hydroxyquinolin-7-yl)phenylmethyl]amino}benzoic acid ethyl ester
Relevant Products
-
4-ACETAMIDO-TEMPO
CAS:14691-89-5
-
UV absorber UV-9
CAS:131-57-7
-
4-Methylbenzyl chloride
CAS:104-82-5