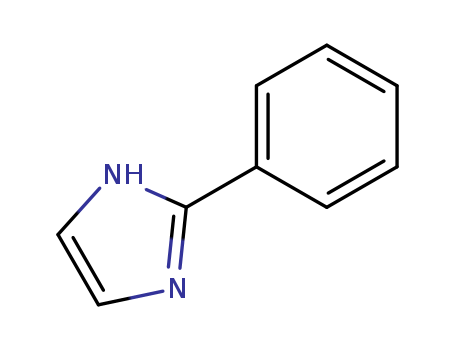
670-96-2
- Product Name:2-Phenylimidazole
- Molecular Formula:C9H8N2
- Purity:99%
- Molecular Weight:144.176
Product Details
Factory Export Top Purity 2-Phenylimidazole 670-96-2 In Stock
- Molecular Formula:C9H8N2
- Molecular Weight:144.176
- Appearance/Colour:colorless to beige chunks or granular powder
- Vapor Pressure:0.000174mmHg at 25°C
- Melting Point:142-148 °C(lit.)
- Refractive Index:1.602
- Boiling Point:340 °C at 760 mmHg
- PKA:13.00±0.10(Predicted)
- Flash Point:180.4 °C
- PSA:28.68000
- Density:1.141 g/cm3
- LogP:2.07670
2-Phenylimidazole(Cas 670-96-2) Usage
InChI:InChI=1/C9H8N2/c1-2-4-8(5-3-1)9-10-6-7-11-9/h1-7H,(H,10,11)
670-96-2 Relevant articles
Nitrene-like Behaviour of Diazoazoles?
Farras, Jaume,Vilarrasa, Jaume
, p. 1127 - 1129 (1986)
Treatment of 4,5-dicyano-2-diazoimidazol...
A sustainable approach towards the three-component synthesis of unsubstituted 1H-imidazoles in the water at ambient conditions
Kapale, Suraj S.,Chaudhari, Hemchandra K.,Mali, Suraj N.,Takale, Balaram S.,Pawar, Hitesh
, p. 712 - 716 (2020/05/22)
A green protocol for the synthesis of un...
Palladium-catalyzed hydrodefluorination of fluoroarenes
Brodney, Michael A.,Gair, Joseph J.,Giroux, Simon,Grey, Ronald L.
, p. 131 - 146 (2021/06/18)
-
Imidazole diarylethene switches: An alternative to acid-gated photochromism
Jiang, Yue,Li, Meng-Lian,Xiong, Kang-Tai,Xu, Hai-Bing,Zeng, Ming-Hua
supporting information, p. 8061 - 8067 (2020/06/10)
We prepared five diarylethenes containin...
Electron Transfer Photoredox Catalysis: Development of a Photoactivated Reductive Desulfonylation of an Aza-Heteroaromatic Ring
Qiang-Liu,Liu, Yu-Xiu,Song, Hong-Jian,Wang, Qing-Min
supporting information, p. 3110 - 3115 (2020/07/04)
Herein, we report a protocol for desulfo...
670-96-2 Process route
-
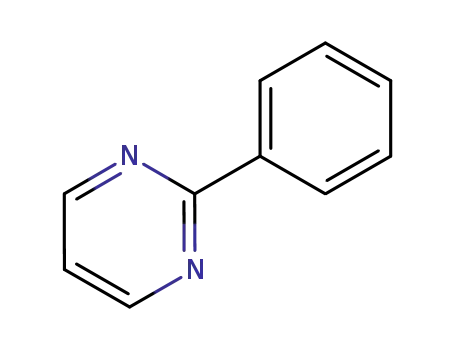
-
7431-45-0
2-phenylpyrimidine

-
-
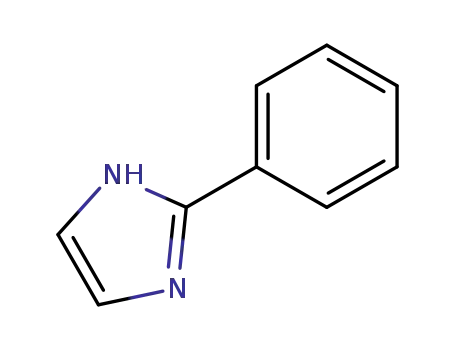
670-96-2
2-Phenylimidazole

-
-
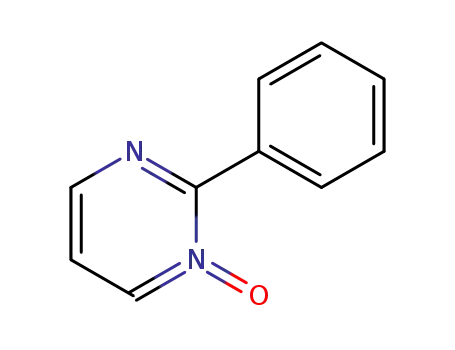
78009-13-9
2-phenylpyrimidine N-oxide

-
-
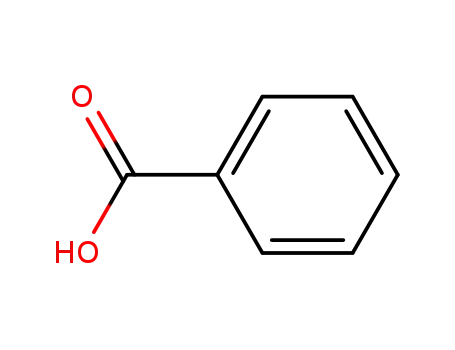
65-85-0,8013-63-6
benzoic acid
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide; acetic acid;
at 70 ℃;
for 6h;
|
5% 2% 6% |
-
-
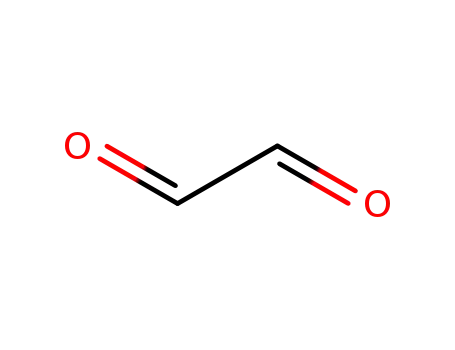
131543-46-9,107-22-2
Glyoxal

-
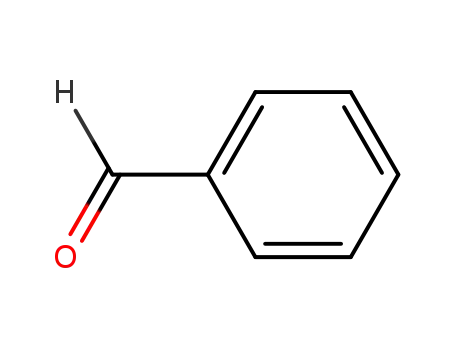
-
100-52-7
benzaldehyde

-

-
670-96-2
2-Phenylimidazole
| Conditions | Yield |
|---|---|
|
With
ammonium acetate; triethylammonium acetate;
In
neat (no solvent);
at 120 ℃;
for 0.666667h;
|
78% |
|
With
ammonium acetate; lipase;
In
water;
at 45 ℃;
for 1h;
Solvent;
Time;
Enzymatic reaction;
|
70% |
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 ℃;
for 0.5h;
|
38% |
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 - 20 ℃;
|
36.8% |
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 - 20 ℃;
|
36.8% |
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 - 20 ℃;
|
36.8% |
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 - 20 ℃;
for 96h;
|
|
|
With
ammonia;
In
water;
at 20 - 80 ℃;
for 12h;
Autoclave;
|
|
|
at 0 - 25 ℃;
for 24h;
|
|
|
With
ammonium hydroxide;
In
ethanol; water;
at 0 - 20 ℃;
for 72h;
|
670-96-2 Upstream products
-
23012-16-0
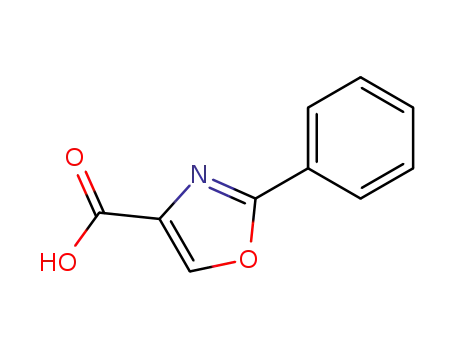
2-phenyl-1,3-oxazole-4-carboxylic acid
-
888-60-8
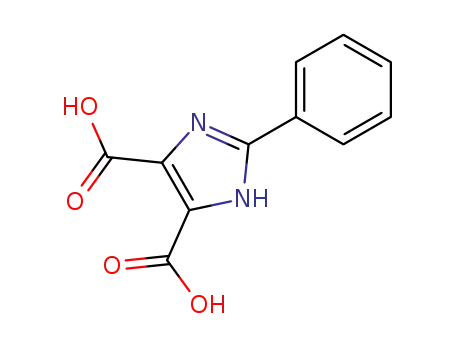
2-phenyl-1H-imidazole-4,5-dicarboxylic acid
-
34039-84-4
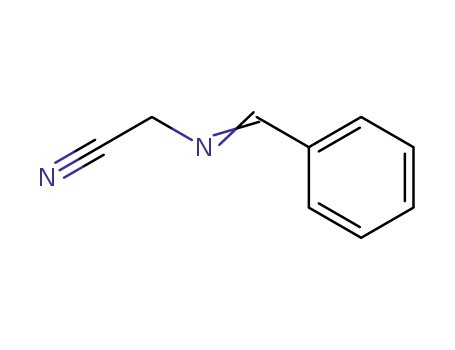
α-(benzylideneamino)acetonitrile
-
645-36-3
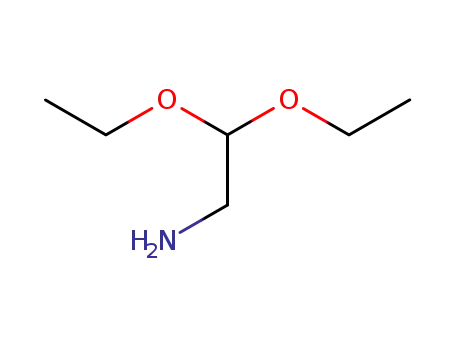
2,2-diethoxy-ethanamine
670-96-2 Downstream products
-
3475-07-8
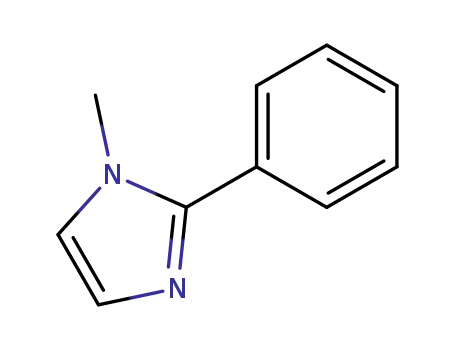
N-methyl-2-phenyl-1H-imidazole
-
14085-43-9
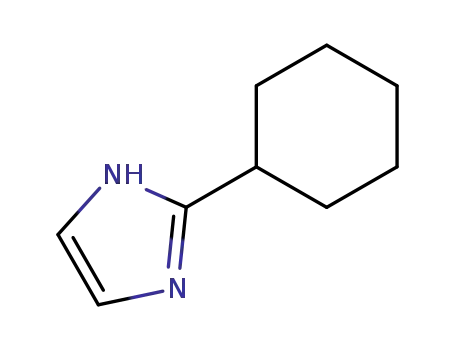
2-cyclohexyl-1H-imidazole
-
121149-51-7
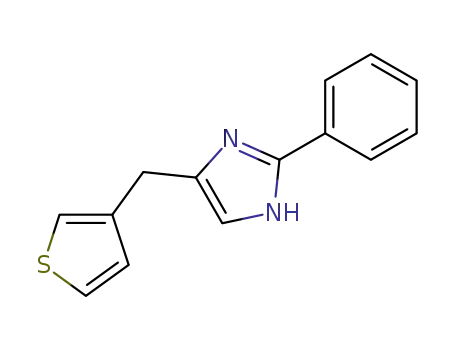
2-Phenyl-4-(3-thienylmethyl)-1H-imidazole
-
121149-53-9
2-phenyl-1-(thien-3-ylmethyl)-1H-imidazole
Relevant Products
-
N,N-Dimethyl-p-toluidine
CAS:99-97-8
-
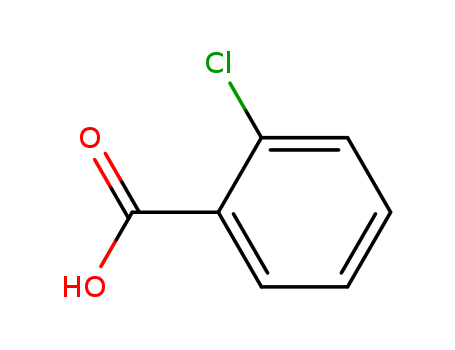
2-Chlorobenzoic acid
CAS:118-91-2
-
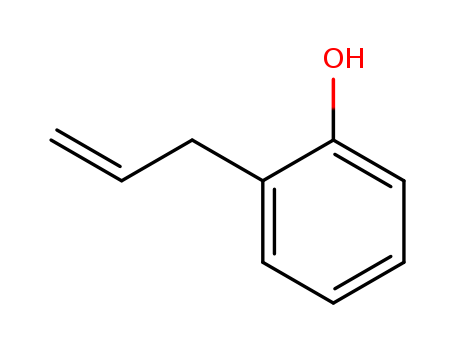
2-Allylphenol
CAS:1745-81-9