118-91-2
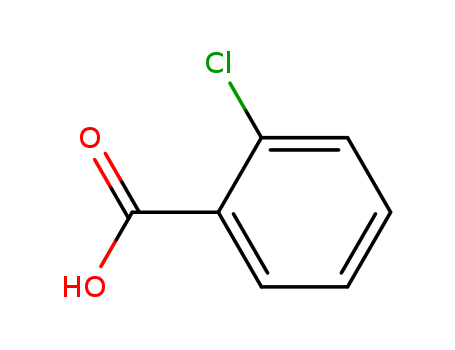
- Product Name:2-Chlorobenzoic acid
- Molecular Formula:C7H5ClO2
- Purity:99%
- Molecular Weight:156.569
Product Details
Quality Factory Supply 99% Pure 2-Chlorobenzoic acid 118-91-2 with Efficient Delivery
- Molecular Formula:C7H5ClO2
- Molecular Weight:156.569
- Appearance/Colour:white crystals or powder
- Vapor Pressure:0.00242mmHg at 25°C
- Melting Point:139-143 °C
- Boiling Point:275.7 °C at 760 mmHg
- Flash Point:120.5 °C
- PSA:37.30000
- Density:1.544 g/cm3
- LogP:2.03820
o-Chlorobenzoic acid(Cas 118-91-2) Usage
|
General Description |
o-Chlorobenzoic acid is a simple organic compound with the chemical formula C7H5ClO2. It consists of a benzene ring bearing a carboxylic acid group and a chlorine atom at the ortho position. o-Chlorobenzoic acid is a white crystalline solid that is used in the synthesis of various pharmaceuticals, dyes, and agrochemicals. It is also used as an intermediate in the production of other chemicals. o-Chlorobenzoic acid is moderately toxic and may cause irritation to the skin, eyes, and respiratory system upon exposure. Additionally, it is not readily biodegradable and it poses a risk to the environment if released into water or soil. |
InChI:InChI=1/C7H5ClO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)/p-1
118-91-2 Relevant articles
-
Atkinson et al.
, p. 476 (1943)
-
TiO2-mediated photomineralization of 2-chlorobiphenyl: The role of O2
Wang, Yongbing,Hong, Chia-Swee
, p. 2791 - 2797 (2000)
Photocatalytic mineralization of 2-chlor...
Oxidation of aromatic aldehydes with potassium bromate-bromide reagent and an acidic catalyst
Sharma,Robert, Alice R.
, p. 3251 - 3254 (2013)
We report herein an easy oxidation proce...
Bismuth(III)-catalyzed oxidative cleavage of aryl epoxides: substituent effects on the kinetics of the oxidation reaction
Boisselier, Veronique Le,Dunach, Elisabet,Postel, Michele
, p. 119 - 124 (1994)
Bismuth(III)mandelate catalyzes the oxid...
Chemiluminescence from arylcarbene oxidation: phenylchlorocarbene and (2-chlorophenyl)carbene
Sander, Wolfram W.
, p. 637 - 646 (1987)
Chemiluminescence is observed in the the...
Supported ruthenium hydride catalysts for direct conversion of alcohols to carboxylic acids using styrene oxide as oxidant
Ghafouri, Moloud,Moghadam, Majid,Mehrani, Kheirollah,Daneshvar, Anahita
, (2018)
In the present work, the ability of two ...
SELECTIVE REMOVAL OF ORTHO HALOGENS BY A DIORGANOLANTHANOID
Deacon, G.B.,MacKinnon, P.I.
, p. 783 - 784 (1984)
Reaction of halogenobenzoic acids with b...
COBALT CARBONYL-CATALYZED DOUBLE-CARBONYLATION OF O-HALOGENATED BENZOIC ACIDS UNDER PHOTOSTIMULATION
Kashimura, Tsugunori,,Kudo, Kiyoshi,Mori, Sadayuki,Sugita, Nobuyuki
, p. 483 - 486 (1986)
Cobalt carbonyl-catalyzed double-carbony...
Reductions and radical cyclizations of aryl and alkyl bromides mediated by NaBH4 in aqueous base
Rai, Roopa,Collum, David B.
, p. 6221 - 6224 (1994)
Reductions and free radical cyclizations...
Direct oxidation of alcohols to carboxylic acids over ruthenium hydride catalyst with diphenyl sulfoxide oxidant
Barati, Behjat,Moghadam, Majid,Rahmati, Abbas,Mirkhani, Valiollah,Tangestaninejad, Shahram,Mohammadpoor-Baltork, Iraj
, p. 114 - 117 (2013)
In the present work, a new method for th...
-
Atkinson et al.
, (1943)
-
Alkali-modified heterogeneous Pd-catalyzed synthesis of acids, amides and esters from aryl halides using formic acid as the CO precursor
Fapojuwo, Dele Peter,Maqunga, Nomathamsanqa Prudence,Meijboom, Reinout,Mogudi, Batsile M.,Molokoane, Pule Petrus,Onisuru, Oluwatayo Racheal,Oseghale, Charles O.
, p. 26937 - 26948 (2021/08/17)
To establish an environmentally friendly...
Efficiency of lithium cations in hydrolysis reactions of esters in aqueous tetrahydrofuran
Hayashi, Kazuhiko,Ichimaru, Yoshimi,Sugiura, Kirara,Maeda, Azusa,Harada, Yumi,Kojima, Yuki,Nakayama, Kanae,Imai, Masanori
, p. 581 - 594 (2021/06/06)
Lithium cations were observed to acceler...
Oxidative carbon-carbon bond cleavage of 1,2-diols to carboxylic acids/ketones by an inorganic-ligand supported iron catalyst
Chen, Weiming,Xie, Xin,Zhang, Jian,Qu, Jian,Luo, Can,Lai, Yaozhu,Jiang, Feng,Yu, Han,Wei, Yongge
supporting information, p. 9140 - 9146 (2021/11/23)
The carbon-carbon bond cleavage of 1,2-d...
Selective oxidation of alkenes to carbonyls under mild conditions
Huo, Jie,Xiong, Daokai,Xu, Jun,Yue, Xiaoguang,Zhang, Pengfei,Zhang, Yilan
supporting information, p. 5549 - 5555 (2021/08/16)
Herein, a practical and sustainable meth...
118-91-2 Process route
-
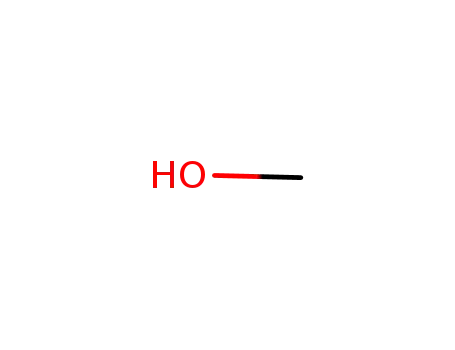
-
67-56-1
methanol

-
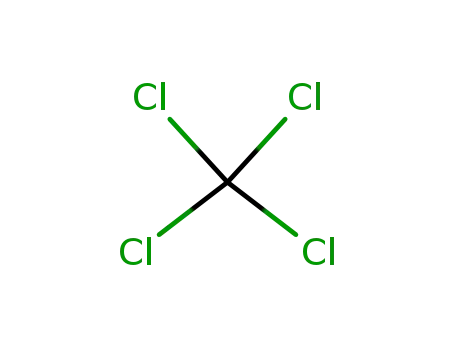
-
56-23-5
tetrachloromethane

-
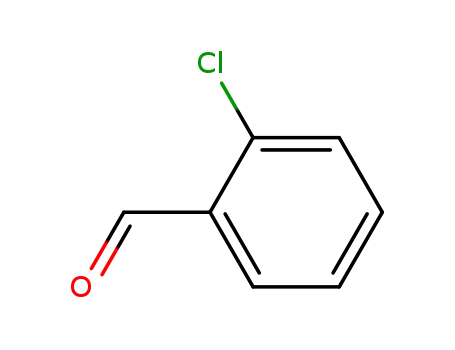
-
89-98-5
2-chloro-benzaldehyde

-
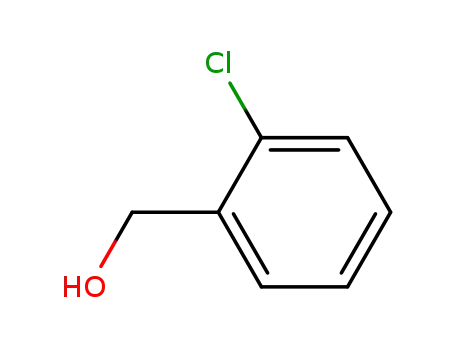
-
17849-38-6
2-Chlorobenzyl alcohol

-
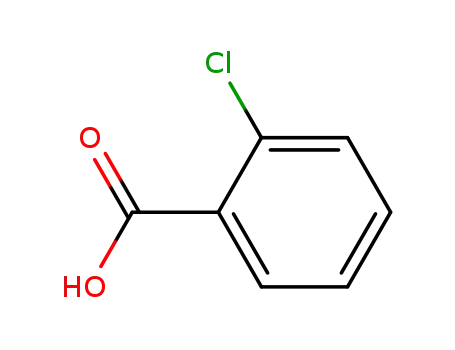
-
118-91-2
ortho-chlorobenzoic acid
| Conditions | Yield |
|---|---|
|
Kinetics;
Disproportionierung;
|
-
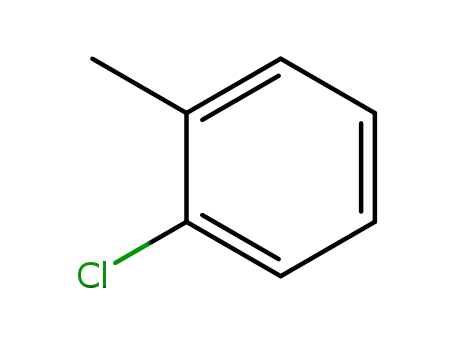
-
95-49-8
2-methylchlorobenzene

-

-
89-98-5
2-chloro-benzaldehyde

-
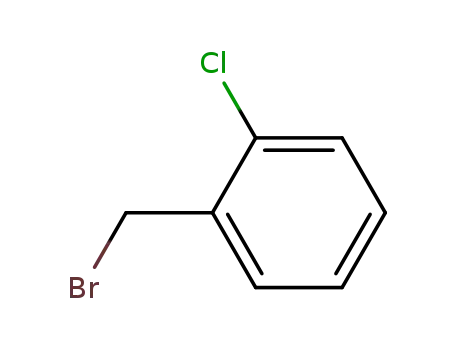
-
611-17-6
1-bromomethyl-2-chlorobenzene

-

-
118-91-2
ortho-chlorobenzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; cobalt(II) acetate; sodium bromide;
In
acetic acid;
at 95 ℃;
for 0.666667h;
Kinetics;
Mechanism;
Rate constant;
other time; other temperature; various concentrations of Co(OAc)2 and NaBr;
|
96% |
|
With
oxygen; cobalt(II) acetate; sodium bromide;
In
acetic acid;
at 95 ℃;
for 0.666667h;
|
95% |
118-91-2 Upstream products
-
873-32-5
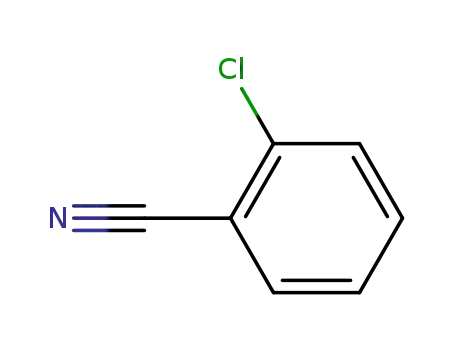
2-Chlorobenzonitrile
-
610-96-8
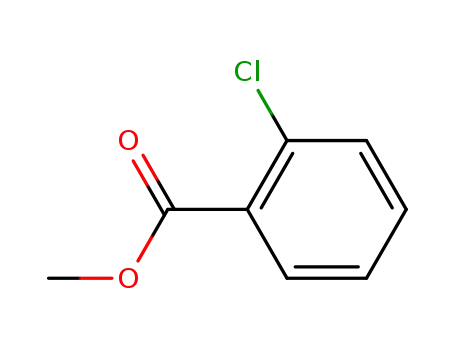
methyl chlorobenzoate
-
65495-21-8
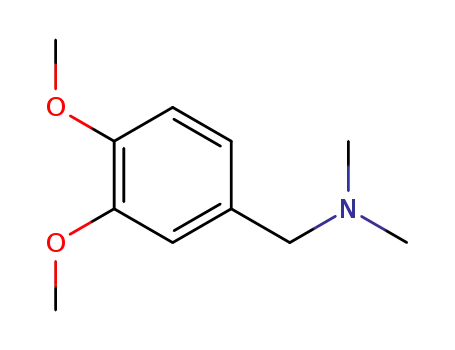
N,N-dimethyl-3,4-dimethoxybenzylamine
-
17333-86-7
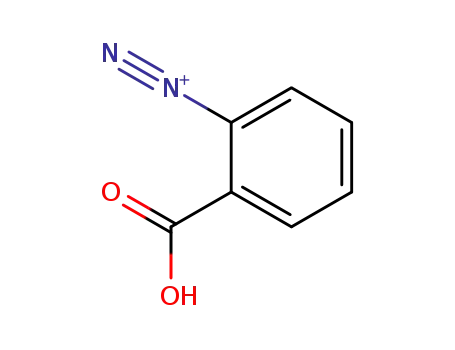
o-Carboxybenzenediazonium
118-91-2 Downstream products
-
28024-48-8
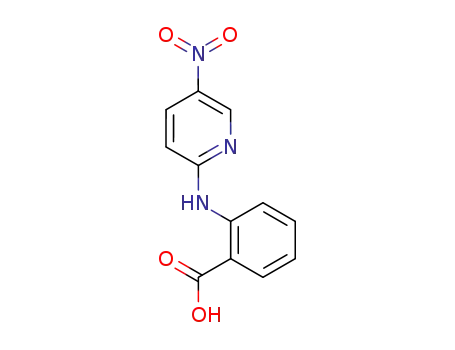
2-(5-nitro-pyridin-2-ylamino)-benzoic acid
-
575-21-3
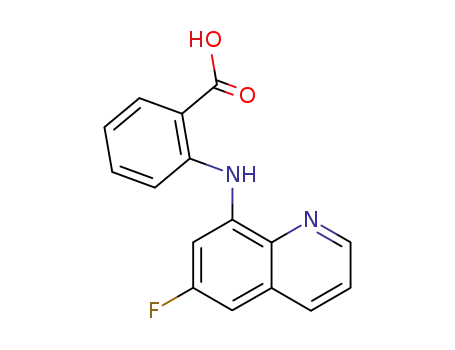
N-(6-fluoro-[8]quinolyl)-anthranilic acid
-
27693-73-8
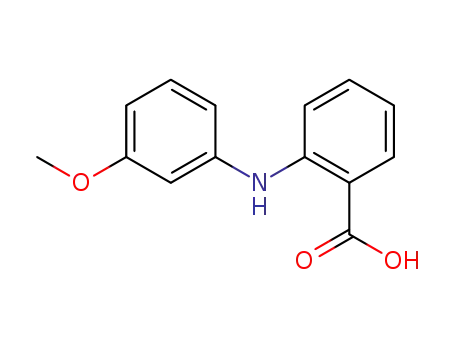
N-(3-methoxyphenyl)anthranilic acid
-
2980-26-9
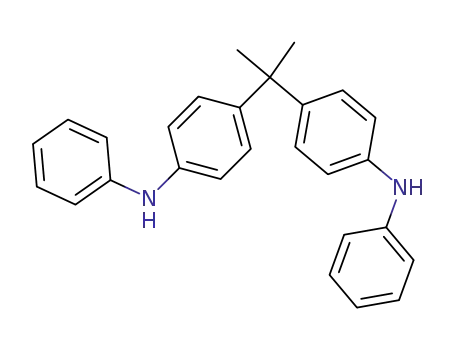
2,2-bis-(4-anilino-phenyl)-propane
Relevant Products
-
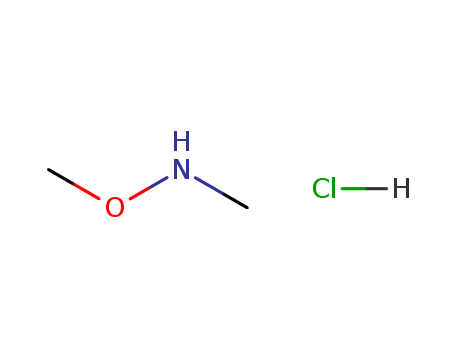
N,O-Dimethylhydroxylamine hydrochloride
CAS:6638-79-5
-
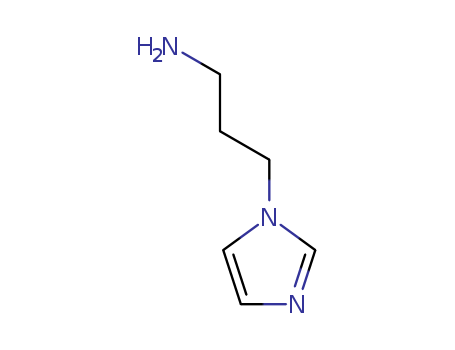
N-(3-Aminopropyl)-imidazole
CAS:5036-48-6
-
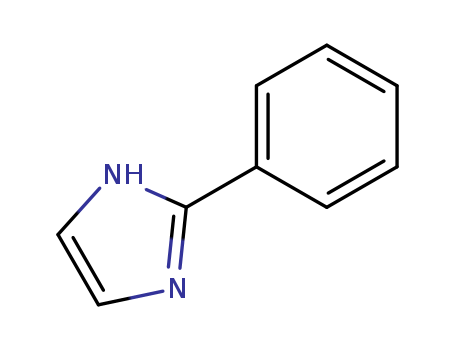
2-Phenylimidazole
CAS:670-96-2