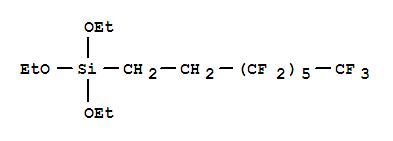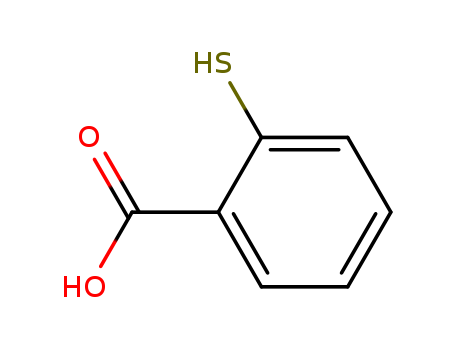
147-93-3
- Product Name:Thiosalicylic acid
- Molecular Formula:C7H6O2S
- Purity:99%
- Molecular Weight:154.189
Product Details
Manufacturer supply good quality Thiosalicylic acid 147-93-3 with stock
- Molecular Formula:C7H6O2S
- Molecular Weight:154.189
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00979mmHg at 25°C
- Melting Point:162-165 °C(lit.)
- Refractive Index:1.5100 (estimate)
- Boiling Point:298.6 °C at 760 mmHg
- PKA:pK1:4.05(0) (20°C)
- Flash Point:134.4 °C
- PSA:76.10000
- Density:1.345 g/cm3
- LogP:1.67350
Thiosalicylic acid(Cas 147-93-3) Usage
|
Chemical Description |
Thiosalicylic acid is a derivative of salicylic acid and is used in the synthesis of various compounds. |
|
Preparation |
Thiosalicylic acid can be prepared from anthranilic acid via diazotization followed by the addition of sodium sulfide and then reduction with zinc. It can also prepared by heating o-halogenated benzoic acids with alkaline hydrosulfide in presence of copper. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 111, p. 654, 1989 DOI: 10.1021/ja00184a038Chemical and Pharmaceutical Bulletin, 33, p. 5184, 1985 DOI: 10.1248/cpb.33.5184 |
|
Purification Methods |
Crystallise the thio acid from hot EtOH (4mL/g), after adding hot distilled water (8mL/g) and boiling with charcoal. The hot solution is filtered, cooled, the solid is collected and dried in vacuo (P2O5). Crystallise it from AcOH and sublime in vacuo.[Beilstein 10 IV 272.] |
|
Application |
thiosalicylic acid can be used as:A nucleophilic trapping agent for the desulfenylation of 3-indolyl sulfides to obtain 3-unsubstituted indoles.A starting material to prepare 2′-mercaptoacetophenone, which is used in the synthesis of thioflavanone by reacting with lithium diisopropylamide and benzaldehyde.A stabilizing agent in the synthesis of metal nanoparticles.It can also be used to prepare 2-thioxanthone-thioacetic acid bimolecular system, which is used as a photoinitiator for free radical polymerization. |
|
Definition |
ChEBI: Thiosalicylic acid is a sulfanylbenzoic acid that is the 2-sulfanyl derivative of benzoic acid. It has a role as a non-narcotic analgesic and an antipyretic. It is a conjugate acid of a thiosalicylate(1-). |
InChI:InChI=1/C7H6O2S/c8-7(9)5-3-1-2-4-6(5)10/h1-4,8-9H/p-2
147-93-3 Relevant articles
-
Katz et al.
, ()
-
Intramolecular Electrostatic and General Acid Catalysis in the Hydrolysis of O,S-Thioacetals
Fife, Thomas H.,Przystas, Theodore J.
, p. 292 - 299 (1980)
The pH-independent release of thiophenol...
First electrospun immobilized molybdenum complex on bio iron oxide nanofiber for green oxidation of alcohols
Noghi, Sedighe Abbaspour,Naeimi, Atena,Hamidian, Hooshang
, p. 229 - 237 (2018)
Bio iron oxide was synthesized from natu...
Scaffold Diversity Inspired by the Natural Product Evodiamine: Discovery of Highly Potent and Multitargeting Antitumor Agents
Wang, Shengzheng,Fang, Kun,Dong, Guoqiang,Chen, Shuqiang,Liu, Na,Miao, Zhenyuan,Yao, Jianzhong,Li, Jian,Zhang, Wannian,Sheng, Chunquan
, p. 6678 - 6696 (2015/09/07)
A critical question in natural product-b...
Efficient Cu-catalyzed base-free C-S coupling under conventional and microwave heating. A simple access to S-heterocycles and sulfides
Soria-Castro, Silvia M.,Penenory, Alicia B.
, p. 467 - 475 (2013/05/08)
S-aryl thioacetates can be prepared by r...
CuI-nanoparticles-catalyzed selective synthesis of phenols, anilines, and thiophenols from aryl halides in aqueous solution
Xu, Hua-Jian,Liang, Yu-Feng,Cai, Zhen-Ya,Qi, Hong-Xia,Yang, Chun-Yan,Feng, Yi-Si
experimental part, p. 2296 - 2300 (2011/06/17)
CuI-nanoparticles-catalyzed selective sy...
147-93-3 Process route
-
![2-phenylbenzo[d]-1,3-oxathiin-4-one](/upload/2025/4/e3c9341c-160a-45eb-aa69-389343a4a87f.png)
-
5651-35-4
2-phenylbenzo[d]-1,3-oxathiin-4-one

-
-
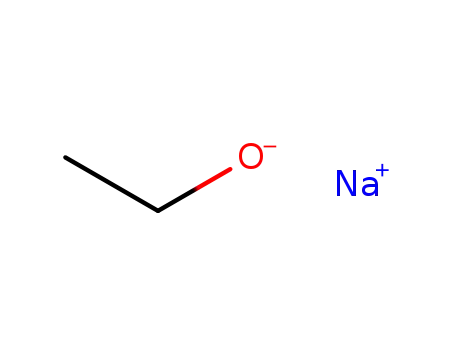
141-52-6
sodium ethanolate

-
-
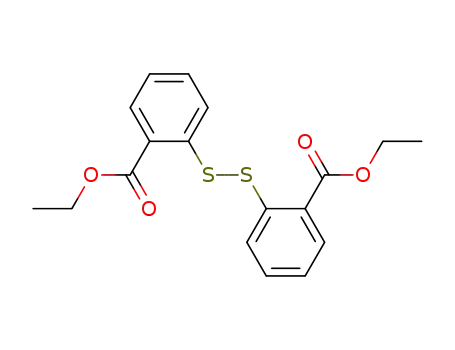
54481-26-4
bis(2-ethoxycarbonylphenyl) disulfide

-
-
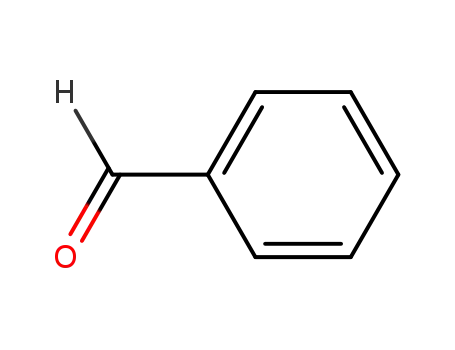
100-52-7
benzaldehyde

-
-
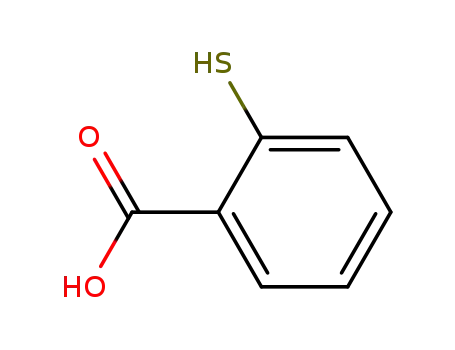
147-93-3
Thiosalicylic acid
| Conditions | Yield |
|---|---|
|
In
benzene;
for 9h;
Heating;
|
46% |
-
![2-phenylbenzo[d]-1,3-oxathiin-4-one](/upload/2025/4/e3c9341c-160a-45eb-aa69-389343a4a87f.png)
-
5651-35-4
2-phenylbenzo[d]-1,3-oxathiin-4-one

-
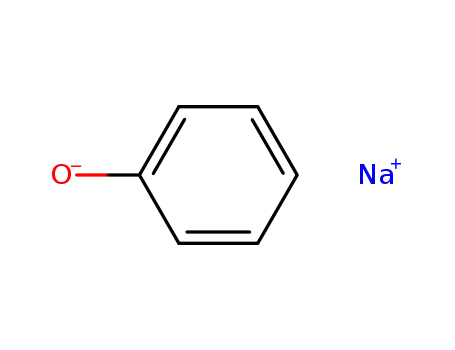
-
139-02-6
sodium phenoxide

-
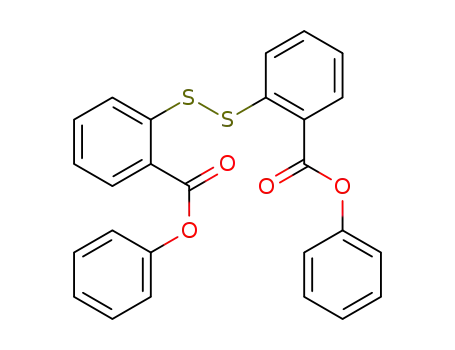
-
29585-74-8
diphenyl 2,2'-disulfanediyldibenzoate

-

-
100-52-7
benzaldehyde

-

-
147-93-3
Thiosalicylic acid
| Conditions | Yield |
|---|---|
|
In
benzene;
for 9h;
Heating;
|
51% |
147-93-3 Upstream products
-
16671-86-6
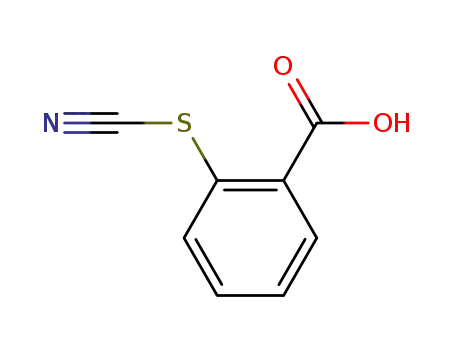
2-thiocyanatobenzoic acid
-
1677-27-6
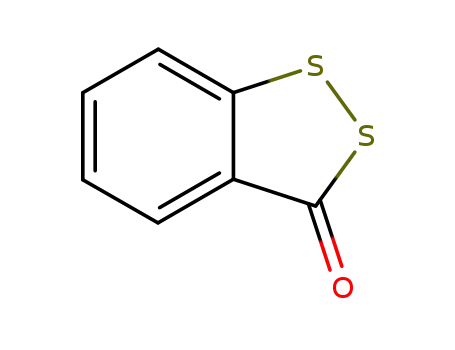
3H-1,2-benzodithiol-3-one
-
20848-83-3
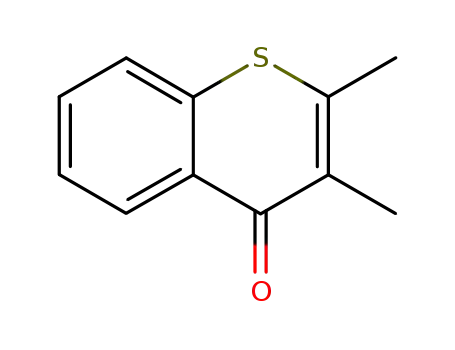
2,2-dimethylthiochromone
-
784-62-3
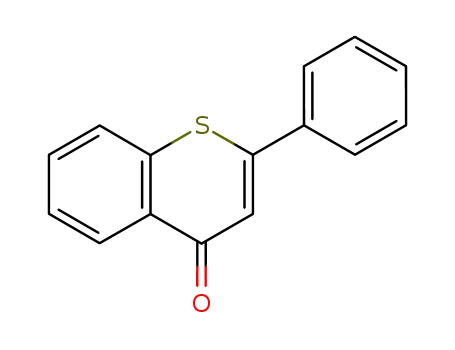
1-thioflavone
147-93-3 Downstream products
-
3704-28-7
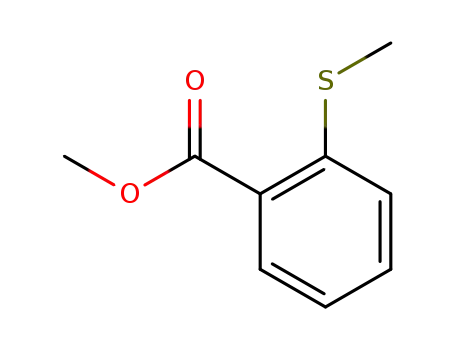
methyl 2-(methylthio)benzoate
-
16233-61-7
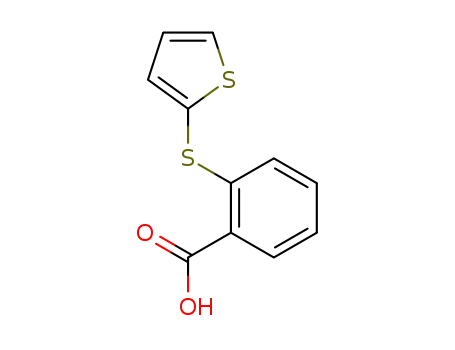
2-thiophen-2-ylsulfanyl-benzoic acid
-
22936-46-5
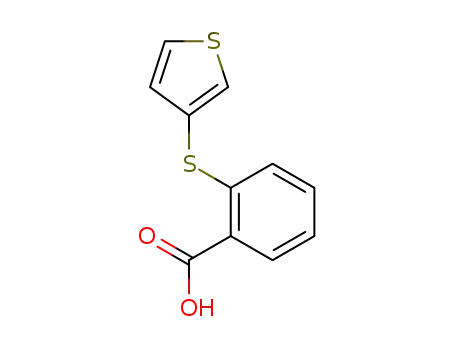
2-[3]thienylmercapto-benzoic acid
-
855470-64-3
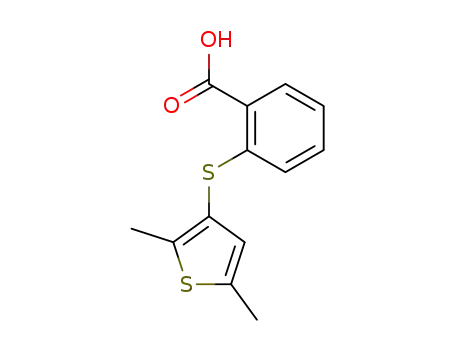
2-(2,5-dimethyl-[3]thienylmercapto)-benzoic acid
Relevant Products
-
1H,1H,2H,2H-Perfluorooctyltriethoxysilane
CAS:51851-37-7
-
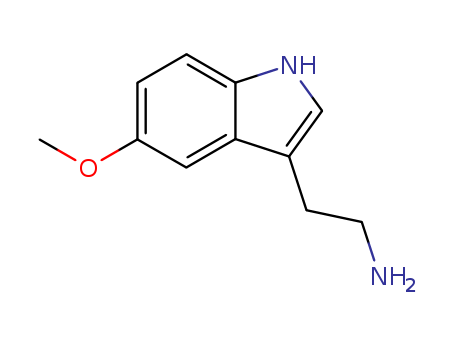
5-Methoxytryptamine
CAS:608-07-1
-
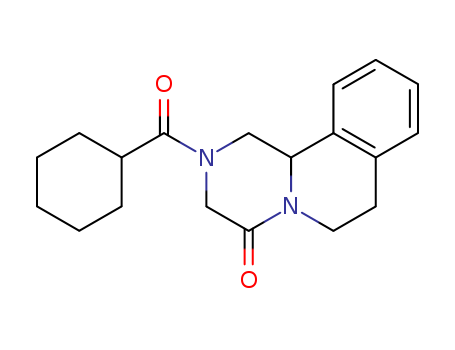
Praziquantel
CAS:55268-74-1