608-07-1
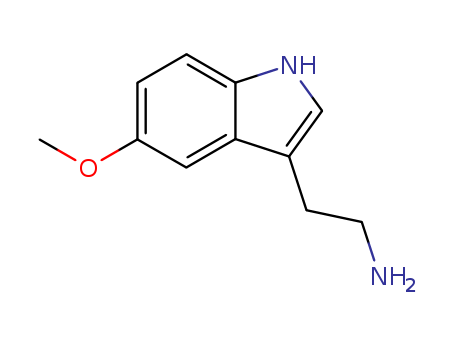
- Product Name:5-Methoxytryptamine
- Molecular Formula:C11H14N2O
- Purity:99%
- Molecular Weight:190.245
Product Details
Quality Factory Hot Selling 5-Methoxytryptamine 608-07-1 with Fast Shipping
- Molecular Formula:C11H14N2O
- Molecular Weight:190.245
- Appearance/Colour:White to tan crystalline powder
- Melting Point:245-250 °C (dec.)(lit.)
- Refractive Index:1.5700 (estimate)
- Boiling Point:380oC
- PKA:16.91±0.30(Predicted)
- PSA:51.04000
- Density:1.0815 (rough estimate)
- LogP:2.37800
5-Methoxytryptamine(Cas 608-07-1) Usage
|
Definition |
ChEBI: 5-methoxytryptamine is a member of the class of tryptamines that is the methyl ether derivative of serotonin. It has a role as a serotonergic agonist, a human metabolite, a mouse metabolite, a 5-hydroxytryptamine 2A receptor agonist, a 5-hydroxytryptamine 2C receptor agonist, a 5-hydroxytryptamine 2B receptor agonist, an antioxidant, a radiation protective agent, a neuroprotective agent and a cardioprotective agent. It is a member of tryptamines, an aromatic ether and a primary amino compound. It derives from a serotonin. It is a conjugate base of a 5-methoxytryptamine(1+). |
|
Application |
5-Methoxytryptamine was used as an agonist in the study of pharmacological profile of the 5-hydroxytryptamine 1 receptor.Reactant for preparation of:Carboline disaccharide domain of shishijimicin AMelatonin analogs for the reduction of intraocular pressure5-HT4 receptor ligandsinhibitors of sortase A and isocitrate lyaseTherapeutic agents for treatment of ischemia/reperfusion (I/R) injuryAurora and epidermal growth factor receptor kinase inhibitorsAgents for the treatment of human papillomavirus infectionManzamine analogues for the control of neuroinflammation and cerebral infectionsInhibitors of pro-inflammatory cytokinesTacrine-melatonin hybrids as multifunctional agents for alzheimer′s disease |
|
General Description |
The protective effect of 5-methoxytryptamine (a metabolite of melatonin) in human keratinocytes against ultraviolet B (UVB) radiation was studied. |
|
Synthesis |
5-Methoxytryptamine (358) was synthesized from 3-(2-iodoethyl)-5-methoxyindole (176) by reaction with 1-methyl-benzylamine (MeCN, 24 h, RT) and subsequent catalytic debenzylation of 44 (H2, Pd/C, EtOH, 24 h, RT, 4 bar). The resulting 5-methoxytryptamine (358 was then reacted with 4-bromobenzoylchloride (THF, NEt3, RT, ON) and the resulting tryptamide 359 was reduced with aluminum hydride to N-(4-bromobenzyl)-5-methoxytryptamine (19) (LiAlH4, AlCl3, Et2O, 5 h, RT), which was isolated as its hydrogen oxalate salt. |
|
Clinical claims and research |
The effects of the 5-HT receptor agonist, 5-methoxytryptamine, on plasma glucose levels were investigated in rats. 5-Methoxytryptamine induced a significant hyperglycemia above the dosage of 1 mg/kg. 5-Methoxytryptamine-induced hyperglycemia was antagonized by pretreatment with the 5-HT1 and 5-HT2 receptor antagonist, methysergide, or the 5-HT2A receptor antagonist, ketanserin, whereas the 5-HT3 and 5-HT4 receptor antagonist, tropisetron, and the 5-HT4 receptor antagonist, SDZ 205-557 (2-methoxy-4-amino-5-chloro-benzoic acid 2-(diethylamino) ethyl ester), showed no effect. In addition, the peripheral 5-HT2 receptor antagonist, xylamidine, reduced 5-methoxytryptamine-induced hyperglycemia. Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: involvement of the peripheral 5-HT2A receptor. |
InChI:InChI=1/C11H11NO3/c1-15-8-2-3-10-9(5-8)7(6-12-10)4-11(13)14/h2-3,5-6,12H,4H2,1H3,(H,13,14)
608-07-1 Relevant articles
Synthesis, pulse radiolysis, and in vitro radioprotection studies of melatoninolipoamide, a novel conjugate of melatonin and α-lipoic acid
Venkatachalam,Salaskar,Chattopadhyay,Barik,Mishra,Gangabhagirathi,Priyadarsini
, p. 6414 - 6419 (2006)
A novel conjugate of melatonin 2 and α-l...
Preparations of melatonin and 1-hydroxymelatonin, and its novel nucleophilic dimerization to (±)-3a,3a'-bispyrrolo[2,3-b]indoles
Somei, Masanori,Oshikiri, Naoki,Hasegawa, Masakazu,Yamada, Fumio
, p. 1237 - 1242 (1999)
A unique synthetic method for melatonin ...
Design, Synthesis, and Biological Activity of Hydrogen Peroxide Responsive Arylboronate Melatonin Hybrids
Bedini, Annalida,Fraternale, Alessandra,Crinelli, Rita,Mari, Michele,Bartolucci, Silvia,Chiarantini, Laura,Spadoni, Gilberto
, p. 100 - 112 (2019)
Stimulus-responsive cleavage reactions h...
Recyclable and reusablen-Bu4NBF4/PEG-400/H2O system for electrochemical C-3 formylation of indoles with Me3N as a carbonyl source
Cheng, Didi,Li, Jingyi,Li, Yujin,Ling, Fei,Liu, Lei,Liu, Tao,Zhong, Weihui
supporting information, p. 4107 - 4113 (2021/06/17)
A safe, practical and eco-friendly elect...
Method for synthesizing melatonin intermediate by taking methoxyphenamine as raw material
-
Paragraph 0023; 0027; 0031, (2021/11/10)
The invention belongs to the technical f...
N-skatyltryptamines-dual 5-ht6r/d2r ligands with antipsychotic and procognitive potential
Bojarski, Andrzej J.,Bugno, Ryszard,Cie?lik, Paulina,Duszyńska, Beata,Handzlik, Jadwiga,Hogendorf, Adam S.,Hogendorf, Agata,Kaczorowska, Katarzyna,Kurczab, Rafa?,Latacz, Gniewomir,Lenda, Tomasz,Sata?a, Grzegorz,Staroń, Jakub,Szewczyk, Bernadeta
, (2021/08/17)
A series of N-skatyltryptamines was synt...
Synthesis method of melatonin
-
Paragraph 0020-0025, (2021/08/07)
The invention discloses a synthesis meth...
608-07-1 Process route
-
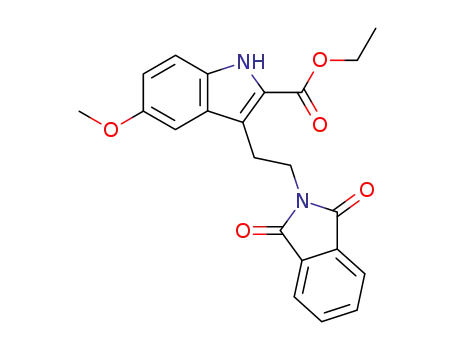
-
55747-53-0
2-ethoxycarbonyl-3-(2-phthalimidoethyl)-5-methoxyindole

-
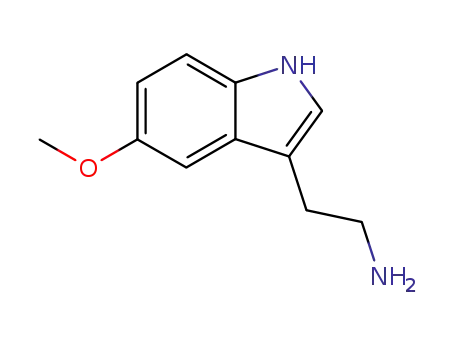
-
608-07-1
2-(5-methoxyindol-3-yl)ethylamine
| Conditions | Yield |
|---|---|
|
2-ethoxycarbonyl-3-(2-phthalimidoethyl)-5-methoxyindole;
With
water; sodium hydroxide;
at 88 - 90 ℃;
With
hydrogenchloride;
In
water;
at 10 - 110 ℃;
Temperature;
|
99% |
|
Multi-step reaction with 2 steps
1: 10 percent aq. NaOH / 0.08 h / Heating; microwave irradiation
2: 20 percent H2SO4 / 0.17 h / Heating; microwave irradiation
With
sodium hydroxide; sulfuric acid;
|
|
|
With
potassium hydroxide;
for 3h;
Reflux;
|
57 g |
-
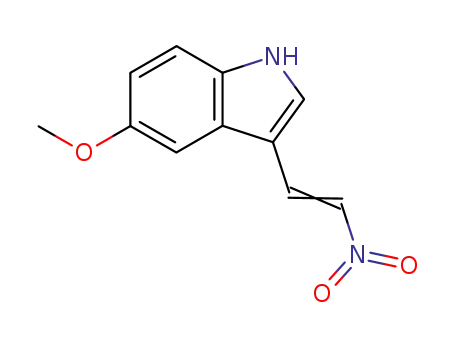
-
61675-19-2
5-methoxy-3-(2-nitroethylenyl)-1H-indole

-

-
608-07-1
2-(5-methoxyindol-3-yl)ethylamine
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 36h;
Inert atmosphere;
|
94% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 40h;
|
73% |
|
With
palladium 10% on activated carbon; hydrogen;
In
tetrahydrofuran;
at 35 ℃;
for 7h;
Reflux;
|
32.2% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
Heating;
|
|
|
5-methoxy-3-(2-nitroethylenyl)-1H-indole;
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
With
water;
In
tetrahydrofuran;
at 0 ℃;
|
|
|
Multi-step reaction with 2 steps
1: sodium tetrahydroborate / tetrahydrofuran; methanol / 20 °C
2: palladium on activated charcoal; hydrogen / methanol / 20 °C
With
sodium tetrahydroborate; palladium on activated charcoal; hydrogen;
In
tetrahydrofuran; methanol;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 20 ℃;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
Reflux;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 36h;
Inert atmosphere;
|
|
|
With
lithium aluminium tetrahydride;
at -78 - 20 ℃;
for 48h;
|
608-07-1 Upstream products
-
1006-94-6
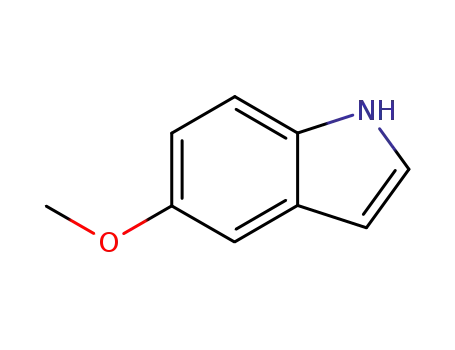
5-methoxylindole
-
917-64-6
methyl magnesium iodide
-
60-29-7
diethyl ether
-
107-14-2
chloroacetonitrile
608-07-1 Downstream products
-
20315-68-8
pinoline
-
102016-76-2
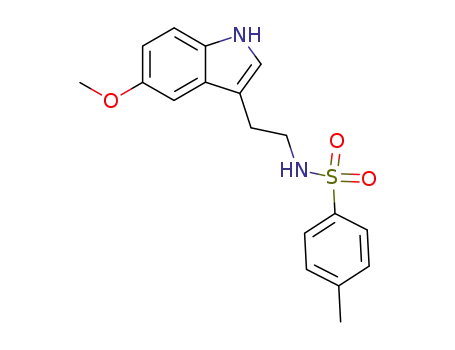
N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide
-
6582-72-5
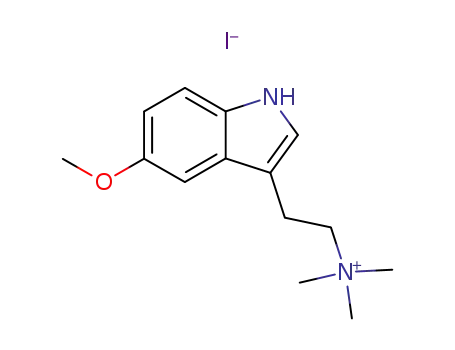
5-Methoxy-N,N-dimethyltryptamin-methiodid
-
50-67-9
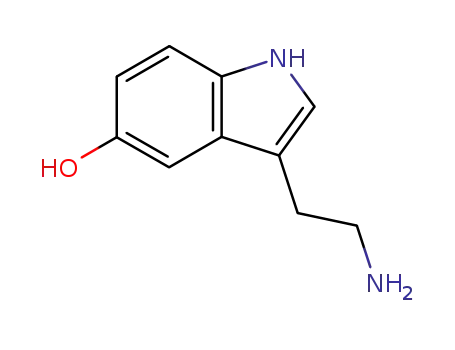
3-(2-aminoethyl)-1H-indol-5-ol
Relevant Products
-
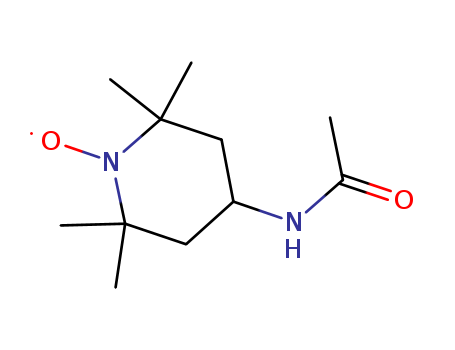
4-ACETAMIDO-TEMPO
CAS:14691-89-5
-
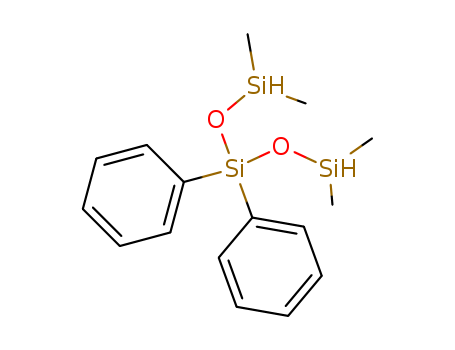
1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane
CAS:17875-55-7
-
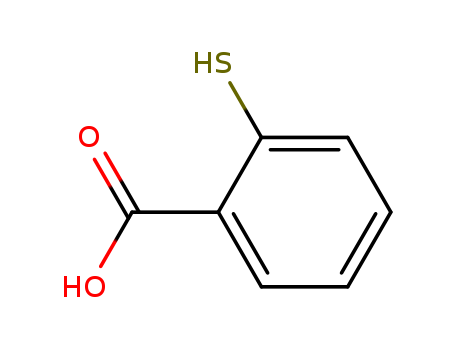
Thiosalicylic acid
CAS:147-93-3