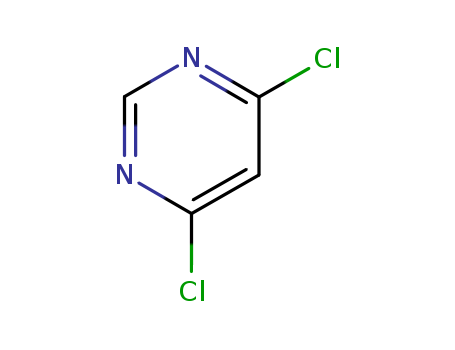
1193-21-1
- Product Name:4,6-Dichloropyrimidine
- Molecular Formula:C4H2Cl2N2
- Purity:99%
- Molecular Weight:148.979
Product Details
Chinese factory supply 4,6-Dichloropyrimidine 1193-21-1 in stock with high standard
- Molecular Formula:C4H2Cl2N2
- Molecular Weight:148.979
- Appearance/Colour:Yellow Solid
- Vapor Pressure:30-390Pa at 20-50℃
- Melting Point:65-67 °C(lit.)
- Refractive Index:1.742
- Boiling Point:176 °C at 760 mmHg
- PKA:-4.20±0.17(Predicted)
- Flash Point:105.1 °C
- PSA:25.78000
- Density:1.493 g/cm3
- LogP:1.78340
4,6-Dichloropyrimidine(Cas 1193-21-1) Usage
|
Synthesis |
The synthesis of 4,6-Dichloropyrimidine is as follows:Dissolve 105.5g of 4,6-diaminopyrimidine in 660.0g of 31% hydrochloric acid and pour into a 2000ml bottle to cool to -5 ° C and dropwise add 500.3g of 33% sodium nitrite. After 2 hours of reaction, HPLC detects 4,6-diamino Pyrimidine is less than 0.5%. 42.8 g of cuprous chloride and 214.0 g of 31% hydrochloric acid are prepared in a 2000 ml bottle. The diazonium salt mother liquor is added dropwise to the bottle. After the dropwise reaction, the reaction is performed at 45 ° C for 2 hours. .Extract with 400g of recovered trichloroethane (200x2 times less than new ones), combine the organic layers for distillation, control the water flush pump 5KPa, temperature 40-140 , collect 404.2g of solvent in the early 40-90 , 90-140 in the later The product was collected at a temperature of 14 ° C to obtain 146.9 g of 4,6-dichloropyrimidine. The yield was 86.4% (based on formazan hydrochloride) and the purity was 99.4%. |
|
General Description |
Cyclic voltammograms of 4,6-dichloropyrimidine shows three cathodic waves, arising from sequential cleavage of carbon-chlorine bonds as well as the reduction of pyrimidine. |
InChI:InChI:1S/C4H2Cl2N2/c5-3-1-4(6)8-2-7-3/h1-2H
1193-21-1 Relevant articles
The efficient one-step chlorination of methylsulfanyl group on pyrimidine ring system with sulfuryl chloride
Ham, Young Jin,Lee, Duck-Hyung,Choi, Hwan Geun,Hah, Jung-Mi,Sim, Taebo
, p. 4609 - 4611 (2010)
A facile one-step transformation of meth...
-
Mantescu et al.
, p. 178,181 (1965)
-
Preparation method of 4, 6-dichloropyrimidine
-
Paragraph 0016; 0030-0045, (2020/08/27)
The invention discloses a preparation me...
Efficient Phosphorus-Free Chlorination of Hydroxy Aza-Arenes and Their Application in One-Pot Pharmaceutical Synthesis
Wang, Jian,Li, Yan-Hui,Pan, Song-Cheng,Li, Ming-Fang,Du, Wenting,Yin, Hong,Li, Jing-Hua
supporting information, p. 146 - 153 (2020/03/10)
The chlorination of hydroxy aza-arenes w...
Synthesis process 4,6-dichloropyrimidine (by machine translation)
-
Paragraph 0015-0017; 0019; 0020, (2020/04/22)
The invention provides a method 4,6 - fo...
Design, synthesis, and anticancer evaluation of acetamide and hydrazine analogues of pyrimidine
Chashoo, Gousia,Khazir, Jabeena,Maqbool, Tariq,Mir, Bilal Ahmad,Pilcher, Lynne,Riley, Darren
, (2020/02/05)
A library of acetamide and hydrazine ana...
1193-21-1 Process route
-
-
C6(2)H6*C4H2Cl2N2

-

-
1193-21-1
4,6-dichloropyrimidine

-
-
1076-43-3
benzene-d6
| Conditions | Yield |
|---|---|
|
In
tetrachloromethane;
at 34.9 ℃;
Equilibrium constant;
|
-

-
1193-24-4
4,6-pyrimidinediol

-

-
1193-21-1
4,6-dichloropyrimidine
| Conditions | Yield |
|---|---|
|
With
triethylamine; trichlorophosphate;
at 40 - 105 ℃;
for 2h;
|
99.1% |
|
With
chlorine; magnesium chloride; phosphorus trichloride;
In
trichlorophosphate;
at 66 ℃;
for 3.5h;
under 750.075 Torr;
Reagent/catalyst;
Temperature;
Pressure;
Solvent;
|
99.83% |
|
With
bis(trichloromethyl) carbonate; sulfuric acid;
In
N,N-dimethyl-formamide; 1,2-dichloro-benzene;
at 80 ℃;
for 4.2h;
Temperature;
Solvent;
Industrial scale;
|
97% |
|
With
cobalt(II) phthalocyanine; bis(trichloromethyl) carbonate; Triphenylphosphine oxide;
In
nitrobenzene;
at 90 - 95 ℃;
for 5h;
Reagent/catalyst;
Temperature;
|
96.2% |
|
With
dmap; thionyl chloride; bis(trichloromethyl) carbonate;
for 7.5h;
Reflux;
Green chemistry;
|
95% |
|
With
thionyl chloride;
In
N,N-dimethyl-aniline;
at 50 ℃;
for 5h;
Temperature;
|
95.6% |
|
With
phosgene; trichlorophosphate;
at 95 - 100 ℃;
for 8h;
Temperature;
Reagent/catalyst;
Large scale;
|
94.9% |
|
With
phosgene; trichlorophosphate;
at 95 - 100 ℃;
for 8h;
Temperature;
Reagent/catalyst;
Large scale;
|
94.9% |
|
With
trichlorophosphate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
90% |
|
With
triethylamine; trichlorophosphate;
at 50 ℃;
for 2h;
Reflux;
|
81.8% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 12h;
Reflux;
|
80% |
|
With
pyridine; phosgene;
In
chloroform;
at 50 ℃;
for 3h;
Concentration;
|
78% |
|
With
trichlorophosphate;
In
water; N,N-dimethyl-aniline; chlorobenzene;
|
58% |
|
With
trichlorophosphate;
at 70 ℃;
for 24h;
|
11% |
|
|
|
|
With
triethylamine; trichlorophosphate;
for 4h;
Reflux;
|
|
|
With
pyridine; phosgene;
In
chloroform;
at 50 ℃;
|
|
|
With
pyridine; phosgene;
In
chloroform;
at 50 ℃;
|
|
|
With
trichlorophosphate;
In
N,N-dimethyl-formamide; toluene;
at 120 ℃;
for 3.5h;
Reflux;
|
|
|
With
phosphorus pentachloride;
at 120 ℃;
|
1193-21-1 Upstream products
-
25286-58-2
1H-pyrimidine-4,6-dione
-
75-44-5
phosgene
-
77287-34-4
formamide
-
75-05-8

acetonitrile
1193-21-1 Downstream products
-
1722-14-1
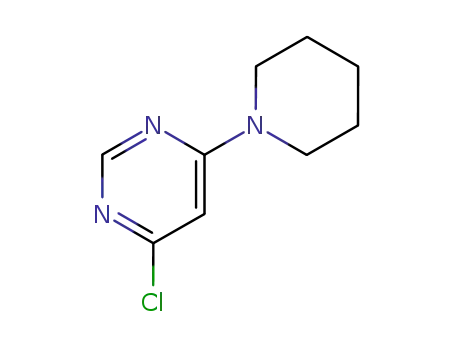
4-Chloro-6-(piperidin-1-yl)pyrimidine
-
26452-81-3
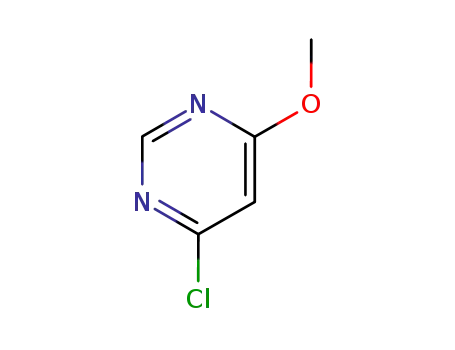
4-chloro-6-methoxy-pyrimidine
-
69591-19-1
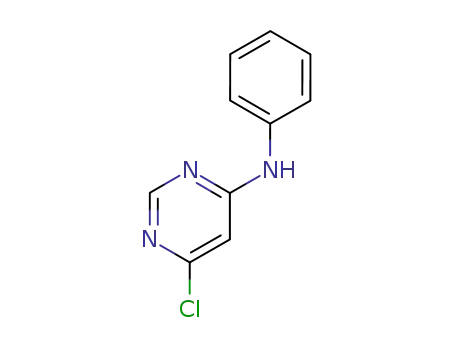
(6-chloropyrimidin-4-yl)phenylamine
-
65766-32-7
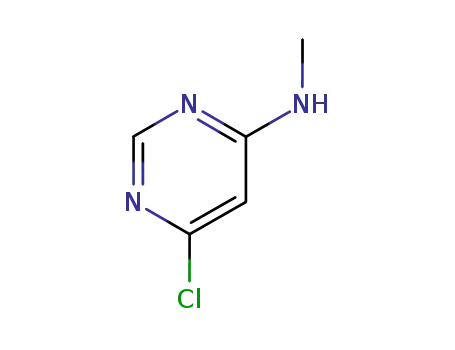
6-chloro-N-methylpyrimidin-4-amine
Relevant Products
-
4-Chloro-o-phenylenediamine
CAS:95-83-0
-
3-MERCAPTO-1-PROPANOL
CAS:19721-22-3
-
4,6-Dihydroxypyrimidine
CAS:1193-24-4