1744-22-5
- Product Name:Riluzole
- Molecular Formula:C8H5F3N2OS
- Purity:99%
- Molecular Weight:234.202
Product Details
Reliable Quality Riluzole 1744-22-5 Hot Sale with Chinese Manufacturer
- Molecular Formula:C8H5F3N2OS
- Molecular Weight:234.202
- Appearance/Colour:white crystalline solid
- Vapor Pressure:0.00145mmHg at 25°C
- Melting Point:116-118 °C
- Boiling Point:296.3 °C at 760 mmHg
- PKA:2.96±0.10(Predicted)
- Flash Point:133 °C
- PSA:76.38000
- Density:1.572 g/cm3
- LogP:3.35830
Riluzole(Cas 1744-22-5) Usage
|
Biological Activity |
Novel psychotropic agent with anticonvulsant, hypnotic, anxiolytic, anti-ischemic and anesthetic properties. Riluzole is able to act as a glutamate release inhibitor, blocks voltage-dependent Na + channels and inhibits GABA uptake by striatal synaptosomes. |
|
Biochem/physiol Actions |
Glutamate release inhibitor; anticonvulsant |
|
Brand name |
Rilutek (Sanofi Aventis). |
InChI:InChI=1/C8H5F3N2OS.ClH/c9-8(10,11)14-4-1-2-5-6(3-4)15-7(12)13-5;/h1-3H,(H2,12,13);1H
1744-22-5 Relevant articles
DNA-binding, -cleavage and antimicrobial investigation on mononuclear Cu(II) Schiff base complexes originated from Riluzole
Daravath, Sreenu,Rambabu, Aveli,Shankar, Dasari Shiva,Shivaraj
, (2021)
Two mononuclear metal complexes, [Cu(L1)...
Intramolecular rearrangement of α-amino acid amide derivatives of 2-aminobenzothiazoles
Pelletier, Jeffrey C.,Velvadapu, Venkata,McDonnell, Mark E.,Wrobel, Jay E.,Reitz, Allen B.
, p. 4193 - 4195 (2014)
We have found that α-amino acid amide de...
DNA interaction, antimicrobial studies of newly synthesized copper (II) complexes with 2-amino-6-(trifluoromethoxy)benzothiazole Schiff base ligands
Rambabu, Aveli,Pradeep Kumar, Marri,Tejaswi, Somapangu,Vamsikrishna, Narendrula,Shivaraj
, (2016)
Four novel Schiff base ligands, L1 (1-((...
Discovery of Potent Carbonic Anhydrase Inhibitors as Effective Anticonvulsant Agents: Drug Design, Synthesis, and in Vitro and in Vivo Investigations
Mishra, Chandra Bhushan,Kumari, Shikha,Angeli, Andrea,Bua, Silvia,Mongre, Raj Kumar,Tiwari, Manisha,Supuran, Claudiu T.
, p. 3100 - 3114 (2021/04/12)
Two sets of benzenesulfonamide-based eff...
An efficient one-pot synthesis of 2-aminobenzothiazoles from substituted anilines using benzyltrimethylammonium dichloroiodate and ammonium thiocyanate in DMSO:H2O
Dass, Reuben,Peterson, Matt A.
supporting information, (2021/10/04)
Treatment of a variety of substituted an...
Iodine-catalyzed amination of benzothiazoles with KSeCN in water to access primary 2-aminobenzothiazoles
Chen, Xiran,Fu, Lianrong,Hao, Xin-Qi,Shi, Linlin,Song, Mao-Ping,Zhu, Xinju,Zhu, Yu-Shen
supporting information, (2021/09/09)
A facile and sustainable approach for th...
1744-22-5 Process route

-
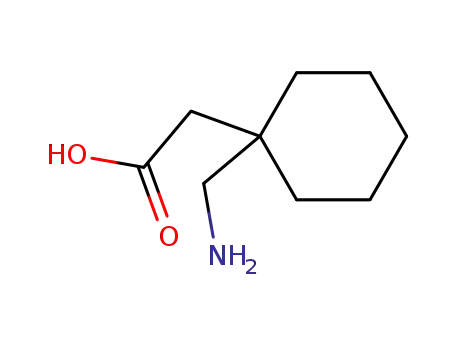
-
60142-96-3
H-Gpn-OH

-
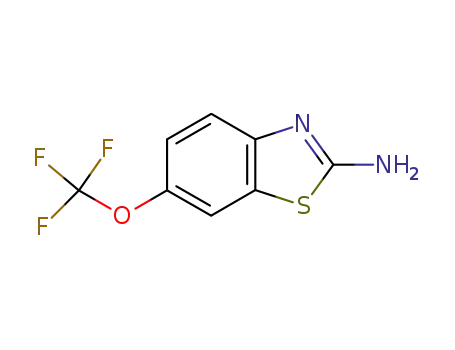
-
1744-22-5
Riluzole
| Conditions | Yield |
|---|---|
|
|
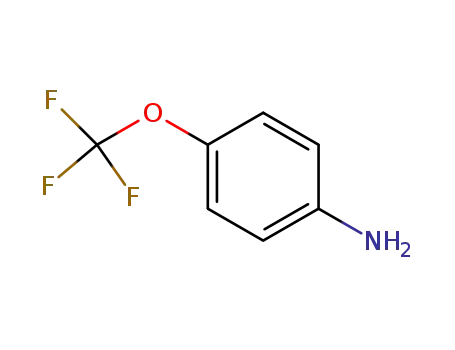
-
-
461-82-5
4-(trifluoromethoxy)aniline

-
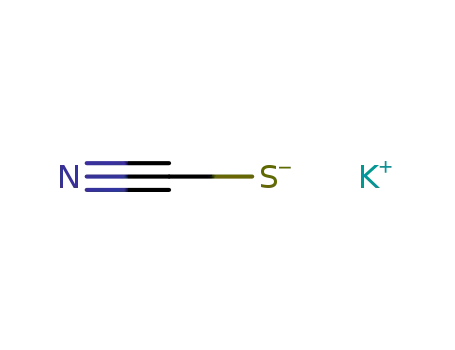
-
333-20-0
potassium thioacyanate

-

-
1744-22-5
Riluzole
| Conditions | Yield |
|---|---|
|
With
bromine; acetic acid;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
95% |
|
4-(trifluoromethoxy)aniline; potassium thioacyanate;
In
acetic acid;
at 20 ℃;
for 0.333333h;
With
bromine;
In
acetic acid;
at 20 ℃;
|
94% |
|
4-(trifluoromethoxy)aniline; potassium thioacyanate;
With
acetic acid;
for 0.166667h;
With
bromine;
|
86% |
|
With
bromine;
In
acetic acid;
at 20 ℃;
Cooling with ice;
|
61% |
|
With
bromine;
In
acetic acid;
for 24h;
|
|
|
With
bromine; acetic acid;
for 21h;
Ambient temperature;
|
|
|
With
bromine; acetic acid;
|
|
|
With
bromine; acetic acid;
at -10 - 0 ℃;
for 4h;
|
|
|
With
bromine; acetic acid;
at 20 ℃;
for 16h;
|
1.8 g |
|
With
bromine; acetic acid;
at 0 - 20 ℃;
|
|
|
With
bromine; acetic acid;
at 0 - 5 ℃;
for 12h;
|
1744-22-5 Upstream products
-
461-82-5

4-(trifluoromethoxy)aniline
-
1147550-11-5

ammonium thiocyanate
-
333-20-0
potassium thioacyanate
-
3674-54-2
tetrabutylammonium thiocyanate
1744-22-5 Downstream products
-
3771-51-5
N,N-dimethyl-4-(6-trifluoromethoxy-benzothiazol-2-ylazo)-aniline
-
130569-75-4
3-(2-hydroxyethyl)-6-trifluoromethoxy-2-benzothiazoline hydrobromide
-
133840-98-9
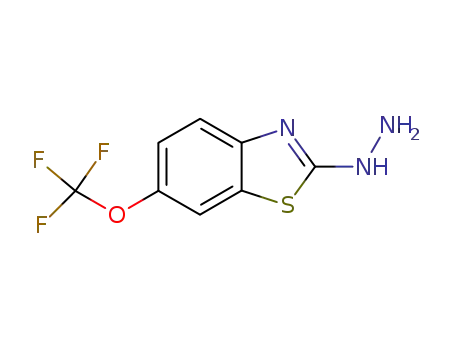
2-hydrazinyl-6-(trifluoromethoxy)benzo[d]thiazole
-
130997-64-7
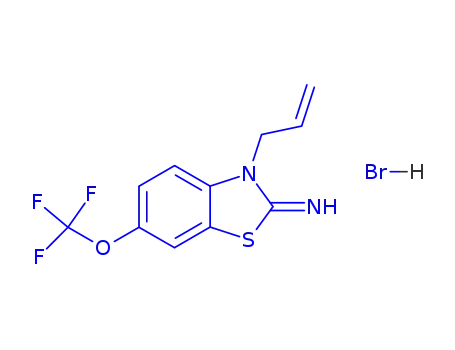
3-allyl-2-imino-6-trifluoromethoxy-benzothiazoline hydrobromide
Relevant Products
-
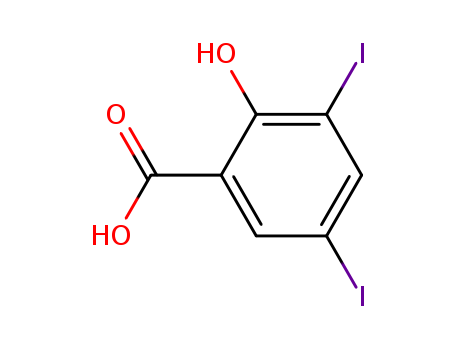
3,5-Diiodosalicylic acid
CAS:133-91-5
-
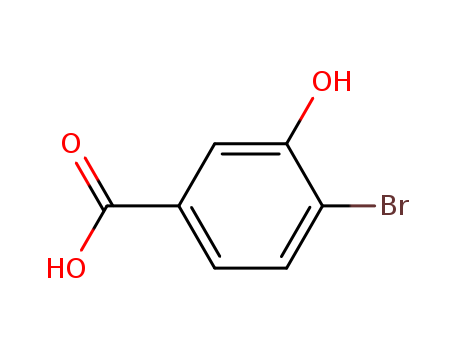
4-BROMO-3-HYDROXYBENZOIC ACID
CAS:14348-38-0