480-40-0
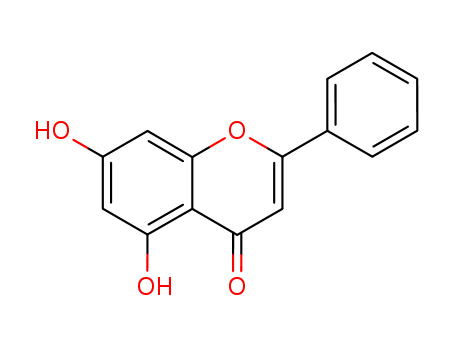
- Product Name:Chrysin
- Molecular Formula:C15H10O4
- Purity:99%
- Molecular Weight:254.242
Product Details
Reputable factory supply Chrysin 480-40-0 in stock with high standard
- Molecular Formula:C15H10O4
- Molecular Weight:254.242
- Appearance/Colour:beige powder
- Vapor Pressure:2.67E-10mmHg at 25°C
- Melting Point:284-286 °C(lit.)
- Refractive Index:1.698
- Boiling Point:491.9 °C at 760 mmHg
- PKA:6.50±0.40(Predicted)
- Flash Point:192.5 °C
- PSA:70.67000
- Density:1.443 g/cm3
- LogP:2.87120
Chrysin(Cas 480-40-0) Usage
|
Chemical Description |
Chrysin is a flavone found in honey and propolis. |
|
Prepration |
The main sources of chrysin are honey and propolis, of which the content of chrysin in honey is 5.3 mg/kg?and that in propolis is 28 g/L. In addition, chrysin is also found in various vegetables, fruits, herbs, and even mushrooms?[10]. Another source of populin is an endophytic fungus Chaetomium globosum, which is related to Chaetomorpha media from India. In addition, chrysin exists in the form of glycosides in walnut, walnut skin, walnut flower and pericarp passion fruit, and in the form of 6-c-arabinoside-8-c-glucoside or glucuronic acid ester in Pinellia ternata. |
|
Physical properties |
beige powder |
|
Definition |
ChEBI: A dihydroxyflavone in which the two hydroxy groups are located at positions 5 and 7. |
InChI:InChI=1/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H
480-40-0 Relevant articles
Oxidation of Flavone, 5-Hydroxyflavone, and 5,7-Dihydroxyflavone to Mono-, Di-, and Tri-Hydroxyflavones by Human Cytochrome P450 Enzymes
Nagayoshi, Haruna,Murayama, Norie,Kakimoto, Kensaku,Tsujino, Masaki,Takenaka, Shigeo,Katahira, Jun,Lim, Young-Ran,Kim, Donghak,Yamazaki, Hiroshi,Komori, Masayuki,Guengerich, F. Peter,Shimada, Tsutomu
, p. 1268 - 1280 (2019)
Biologically active plant flavonoids, in...
Continuous flow microchannel synthesis process of flavonoid compounds
-
Paragraph 0050-0060, (2021/06/22)
The invention provides a continuous flow...
Novel chromenone derivatives having substituted biphenyl group and a pharmaceutical composition for prevention or treatment of allergic diseases compring the same
-
, (2020/11/26)
The present invention relates to: a nove...
Scope and Mechanism of the Ruthenium-Catalyzed Dehydrative C-H Coupling of Phenols with α,β-Unsaturated Carbonyl Compounds: Expedient Synthesis of Chromene and Benzoxacyclic Derivatives
Mokar, Bhanudas Dattatray,Yi, Chae S.
, p. 4625 - 4632 (2019/12/24)
Chromene and benzoxacyclic derivatives w...
480-40-0 Process route
-
-
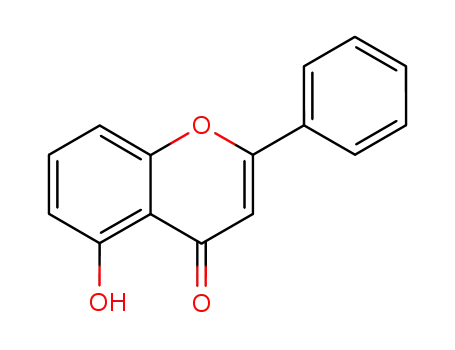
491-78-1,66585-06-6
5-Hydroxyflavone

-
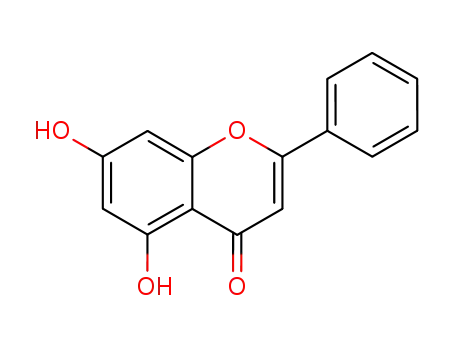
-
480-40-0
5,7-dihydroxy-2-phenyl-chromen-4-one

-
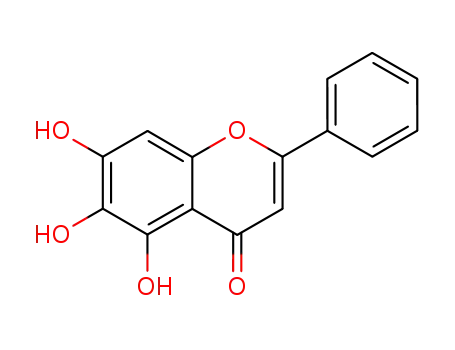
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one
| Conditions | Yield |
|---|---|
|
5-Hydroxyflavone;
at 37 ℃;
for 0.333333h;
With
NADP; D-glucose-6-
|
-
-
1350535-30-6
chrysin 7-O-α-L-arabinopyranosyl-(1->6)-β-D-glucopyranoside

-
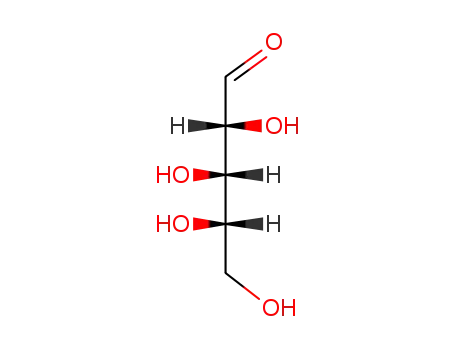
-
5328-37-0
L-arabinose

-
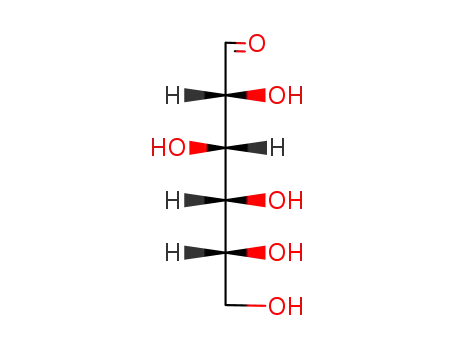
-
50-99-7
D-glucose

-

-
480-40-0
5,7-dihydroxy-2-phenyl-chromen-4-one
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; water;
In
methanol;
for 5h;
Heating;
|
6 mg |
480-40-0 Upstream products
-
480-39-7
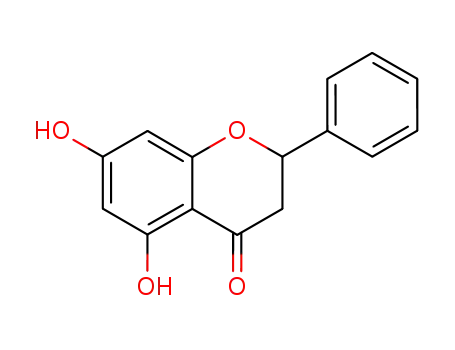
pinocembrin
-
273211-30-6
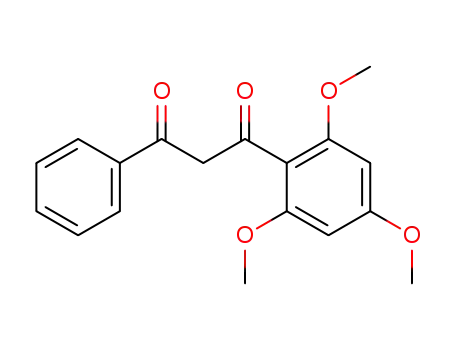
1-phenyl-3-(2,4,6-trimethoxy-phenyl)-propane-1,3-dione
-
108-73-6
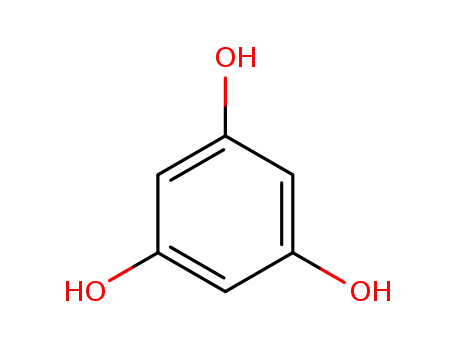
3,5-dihydroxyphenol
-
94-02-0

ethyl 3-oxo-3-phenylpropionate
480-40-0 Downstream products
-
520-28-5
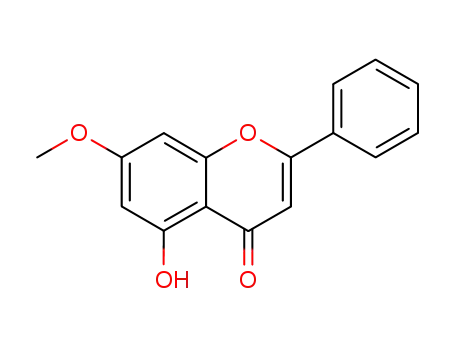
tectochrysine
-
109817-90-5
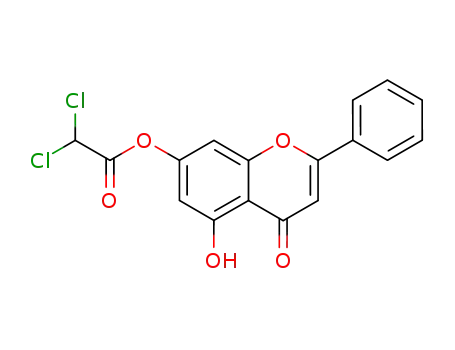
7-dichloroacetoxy-5-hydroxy-2-phenyl-chromen-4-one
-
6674-40-4
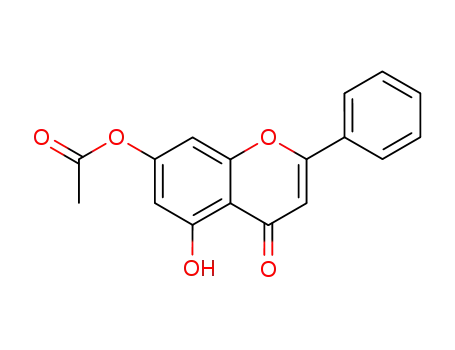
5-hydroxy-7-acetoxyflavone
-
6665-78-7
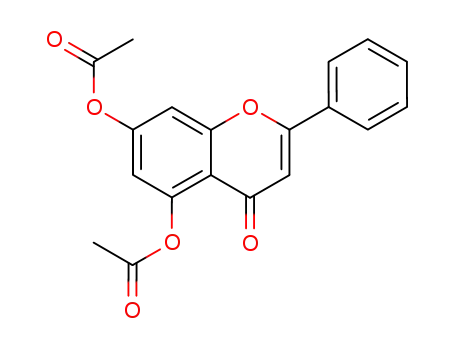
5,7-di-O-acetyl chrysin
Relevant Products
-
Ethoxylated trimethylolpropane triacrylate
CAS:28961-43-5
-
Intermediate
CAS:434-16-2
-
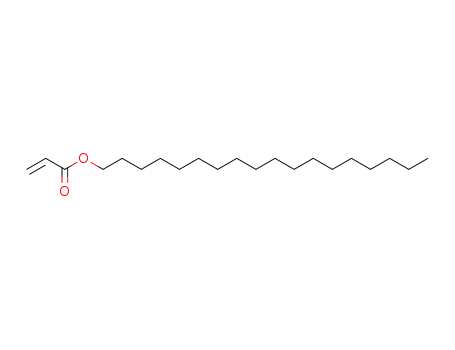
Octadecyl acrylate
CAS:4813-57-4