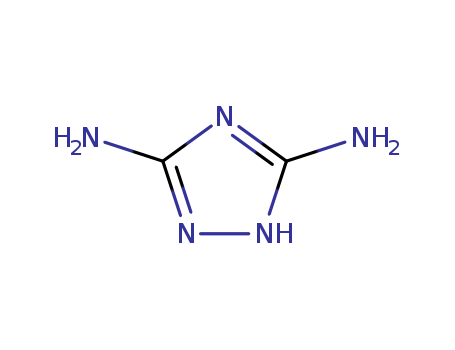
1455-77-2
- Product Name:1H-1,2,4-Triazole-3,5-diamine
- Molecular Formula:C2H5N5
- Purity:99%
- Molecular Weight:99.0952
Product Details
Quality Factory Sells Top Purity 99% 1H-1,2,4-Triazole-3,5-diamine 1455-77-2 with Safe Delivery
- Molecular Formula:C2H5N5
- Molecular Weight:99.0952
- Appearance/Colour:colourless crystals or faintly yellow powder
- Vapor Pressure:3.85E-09mmHg at 25°C
- Melting Point:202-205 °C(lit.)
- Refractive Index:1.809
- Boiling Point:473.7 °C at 760 mmHg
- PKA:12.10±0.40(Predicted)
- Flash Point:271.6 °C
- PSA:93.61000
- Density:1.686 g/cm3
- LogP:0.13150
1H-1,2,4-Triazole-3,5-diamine(Cas 1455-77-2) Usage
|
Definition |
ChEBI: An aromatic amine that is 1,2,4-triazole substituted at positions 3 and 5 by amino groups. |
|
General Description |
Colorless crystals. |
|
Air & Water Reactions |
1H-1,2,4-Triazole-3,5-diamine is sensitive to air. Dust can be explosive when suspended in air at specific concentrations. Water soluble. |
|
Reactivity Profile |
The triazoles, of which 1H-1,2,4-Triazole-3,5-diamine is a member, are a group of highly explosive materials that are sensitive to heat, friction, and impact. Sensitivity varies with the type of substitution to the triazole ring. The amine substituted derivatives, 1H-1,2,4-Triazole-3,5-diamine, tend not to be explosion sensitive. Metal chelated and halogen substitution of the triazol ring make for a particularly heat sensitive material. Azido and nitro derivatives have been employed as high explosives. No matter the derivative these materials should be treated as explosives. 1H-1,2,4-Triazole-3,5-diamine forms salts readily with acids. |
|
Health Hazard |
SYMPTOMS: 1H-1,2,4-Triazole-3,5-diamine is stable under normal laboratory conditions. |
|
Fire Hazard |
Flash point data for 1H-1,2,4-Triazole-3,5-diamine are not available. 1H-1,2,4-Triazole-3,5-diamine is probably combustible. |
|
Safety Profile |
Human systemic effects by intravenous route: leukopenia (reduced white blood cell count) and thrombo cytopenia (reduced blood platelet count). Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
The triazole crystallises from water or EtOH. [Beilstein 26 III/IV 1161.] |
InChI:InChI=1/C2H5N5/c3-1-5-2(4)7-6-1/h(H5,3,4,5,6,7)
1455-77-2 Relevant articles
Efficient in situ synthesis of 3,5-disubstituted-1,2,4-triazoles under microwave-assisted conditions
Wei, Qing,Qiao, Chengfang,Xia, Zhengqiang,Chen, Sanping
, p. 3181 - 3191 (2013)
Abstract Based on an efficient in situ m...
Preparation and characterization of 3,5-dinitro-1H-1,2,4-triazole
Haiges,Bélanger-Chabot,Kaplan,Christe
, p. 7586 - 7594 (2015)
Neat 3,5-dinitro-1H-1,2,4-triazole was o...
Technology for three-innovation synthesis of 2-amino-5-methyl-4-oxo-3-n-propyltriazolopyrimidine
-
Paragraph 0015; 0029, (2019/11/29)
The invention discloses a technology for...
Silica Metal Oxide Vesicles Catalyze Comprehensive Prebiotic Chemistry
Mattia Bizzarri, Bruno,Botta, Lorenzo,Pérez-Valverde, Maritza Iveth,Saladino, Raffaele,Di Mauro, Ernesto,García-Ruiz, Juan Manuel
, p. 8126 - 8132 (2018/05/29)
It has recently been demonstrated that m...
Hydrothermal synthesis method of 3,5-diamino-1,2,4-triazole
-
Paragraph 0073; 0074; 0075, (2018/02/04)
The invention provides a hydrothermal sy...
1455-77-2 Process route
-
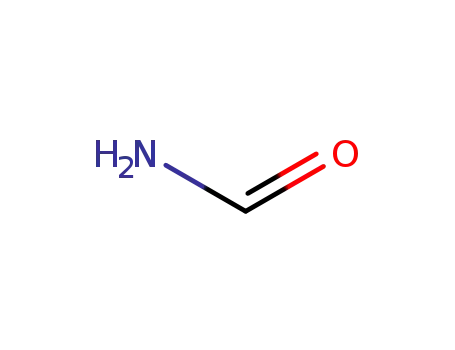
-
77287-34-4,77287-35-5,60100-09-6
formamide

-
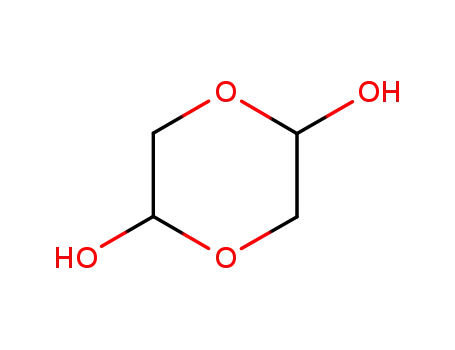
-
23147-58-2,110822-84-9,110822-85-0,77431-49-3
glycolaldehyde dimer

-
-
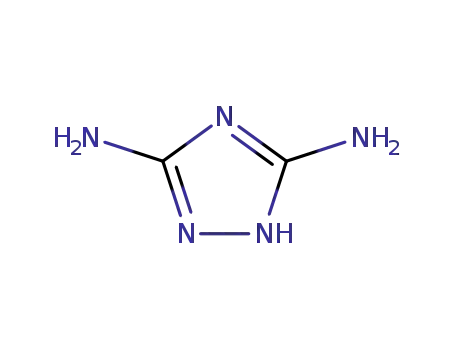
1455-77-2
guanazole

-

-
849585-22-4,106989-11-1,26811-96-1,31587-11-8,26100-51-6
LACTIC ACID

-
-
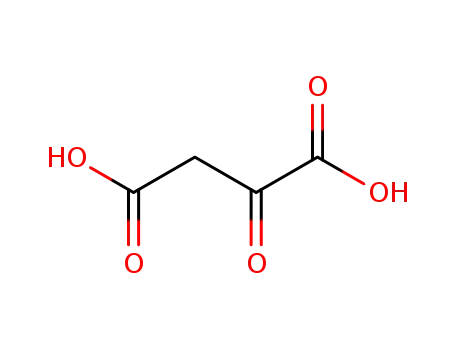
328-42-7
Oxalacetic acid

-
-
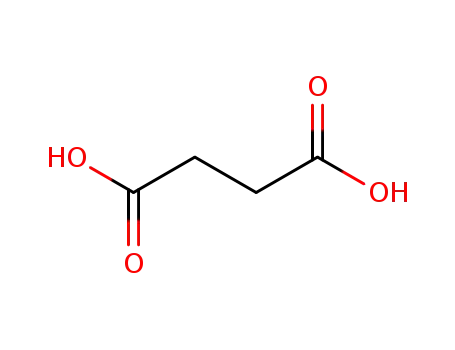
110-15-6
succinic acid

-
-
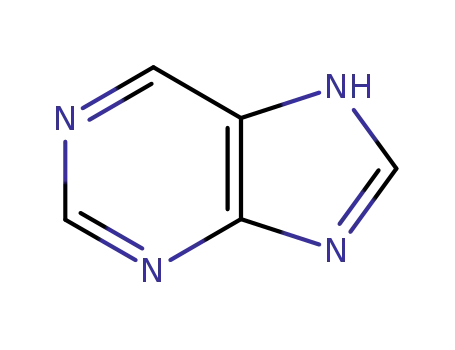
120-73-0,149297-77-8,51953-03-8
purine

-
-
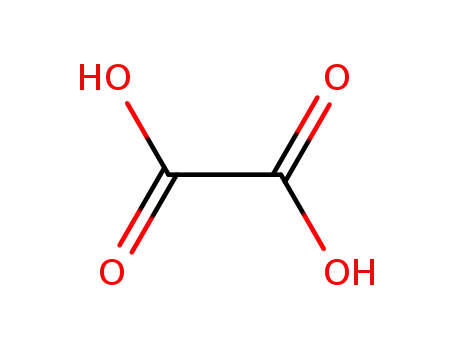
144-62-7,97993-78-7
oxalic acid

-
-
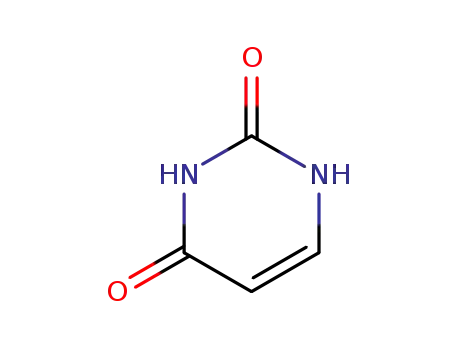
66-22-8,144104-68-7,18324-22-6,27072-01-1,2920-92-5,51953-19-6
uracil

-
-
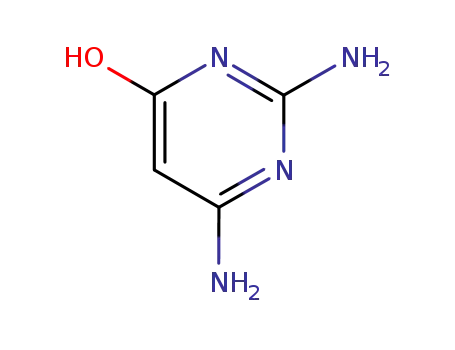
56-06-4
2,6-diaminopyrimidin-4-ol

-
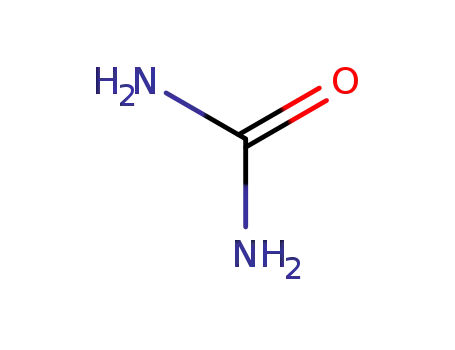
-
57-13-6
urea
| Conditions | Yield |
|---|---|
|
With
copper(II) chloride tetrahydrate; water;
at 80 ℃;
for 24h;
|
-

-
77287-34-4,77287-35-5,60100-09-6
formamide

-
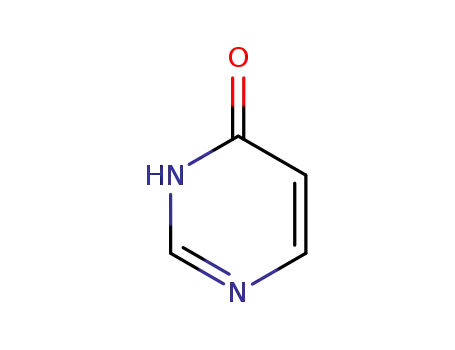
-
51953-18-5,542-27-8
pyrimidine-4(3H)-one

-

-
1455-77-2
guanazole

-
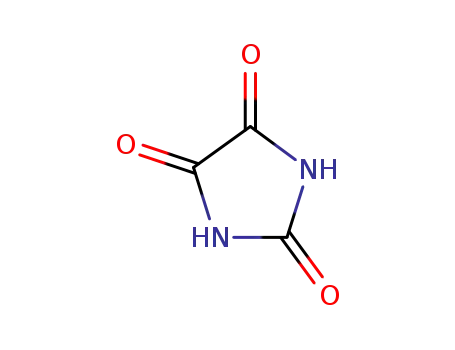
-
120-89-8
parabanic acid

-

-
849585-22-4,106989-11-1,26811-96-1,31587-11-8,26100-51-6
LACTIC ACID

-
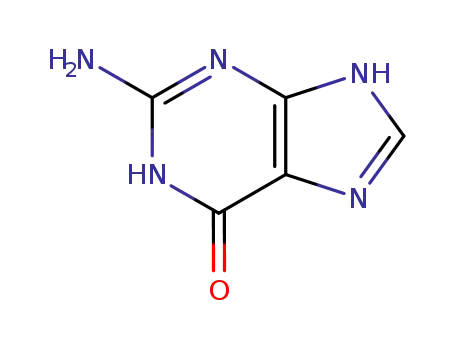
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-

-
328-42-7
Oxalacetic acid

-
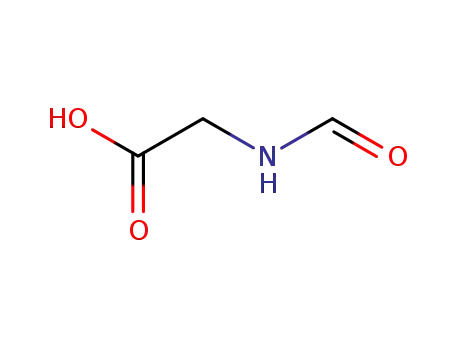
-
2491-15-8
formylglycine

-

-
110-15-6
succinic acid

-
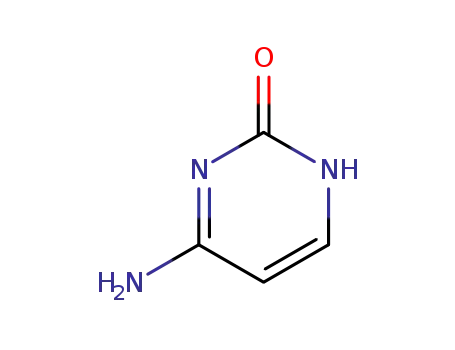
-
71-30-7
Cytosine

-

-
120-73-0,149297-77-8,51953-03-8
purine

-

-
144-62-7,97993-78-7
oxalic acid

-
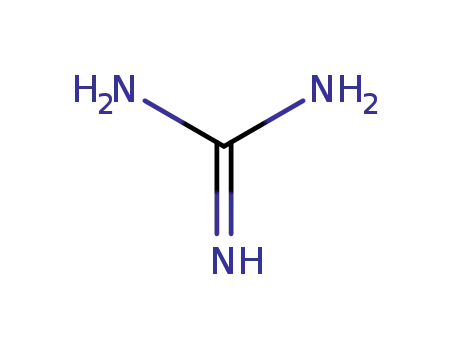
-
113-00-8
guanidine nitrate

-
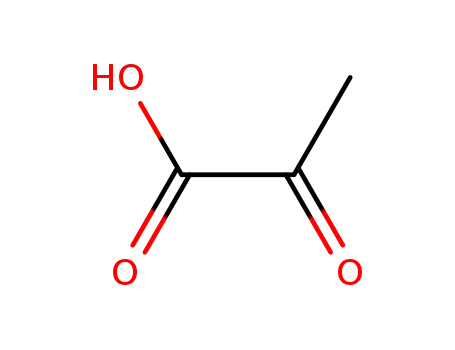
-
127-17-3
2-oxo-propionic acid

-

-
66-22-8,144104-68-7,18324-22-6,27072-01-1,2920-92-5,51953-19-6
uracil

-

-
56-06-4
2,6-diaminopyrimidin-4-ol

-
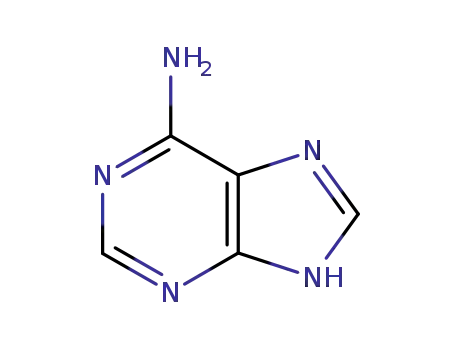
-
66224-66-6,66224-67-7,66224-68-8,66224-69-9,134434-48-3,134434-49-4,134454-76-5,134461-75-9
adenine

-

-
57-13-6
urea

-
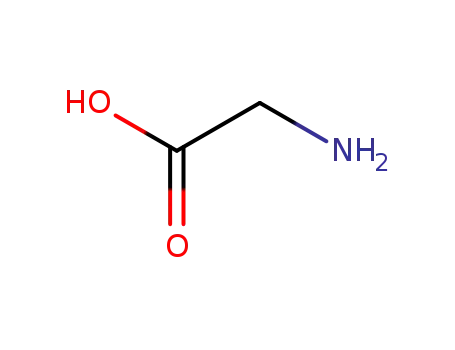
-
56-40-6,18875-39-3,25718-94-9
glycine

-
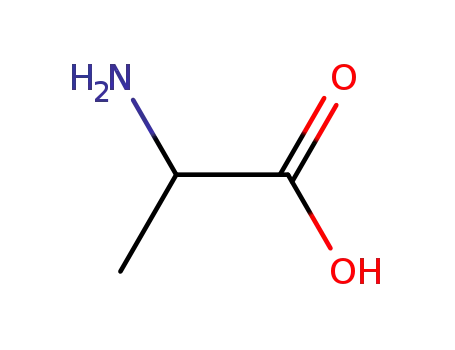
-
302-72-7,25191-17-7,18875-37-1
rac-Ala-OH

-
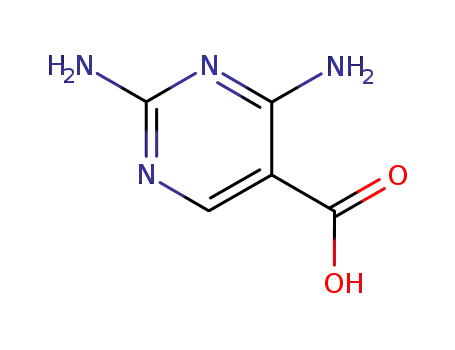
-
18588-61-9
2,4-diamino-pyrimidine-5-carboxylic acid

-
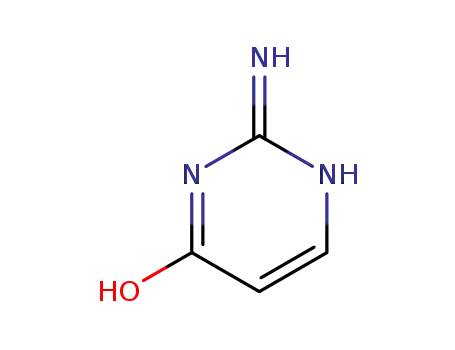
-
155831-93-9,176773-00-5,176799-47-6
isocytosine
| Conditions | Yield |
|---|---|
|
With
ferric sulfate nonahydrate; water;
at 80 ℃;
for 24h;
|
1455-77-2 Upstream products
-
127099-85-8
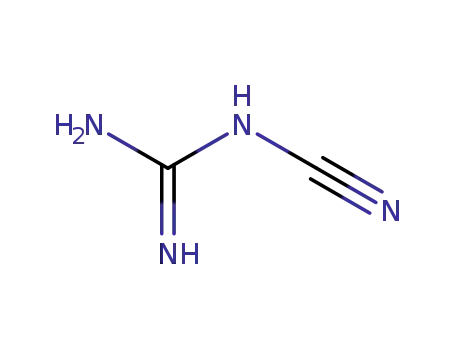
N-Cyanoguanidine
-
131023-09-1
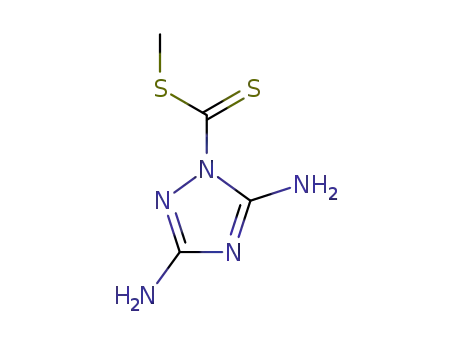
methyl (3,5-diamino-1,2,4-triazol-1-yl)dithiocarbonate
-
60-34-4
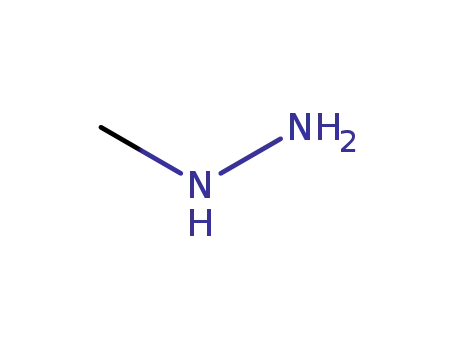
methylhydrazine
-
64-17-5
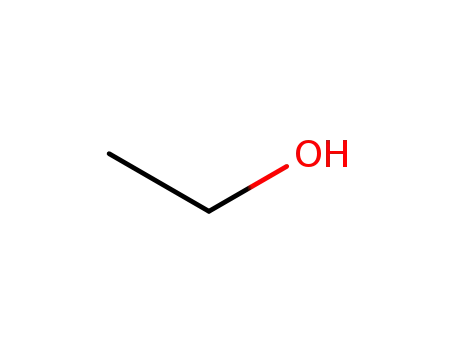
ethanol
1455-77-2 Downstream products
-
99969-13-8
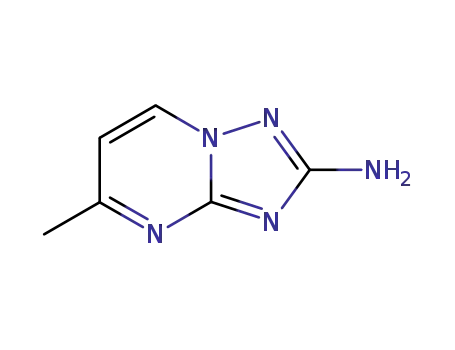
5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-ylamine
-
72436-99-8
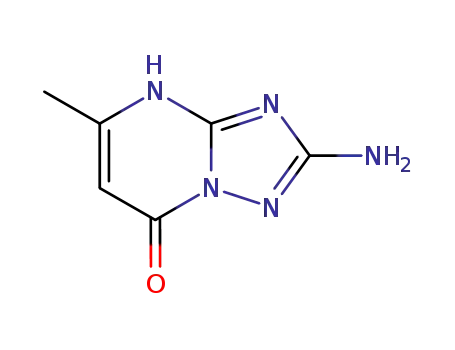
2-amino-5-methyl-4H-[1,2,4]triazolo[1,5-a]pyrimidin-7-one
-
62176-89-0
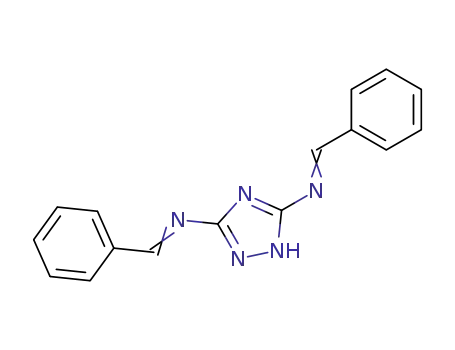
N,N'-dibenzylidene-1H-[1,2,4]triazole-3,5-diamine
-
62176-79-8
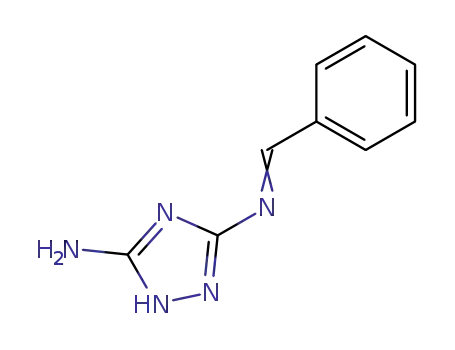
N-benzylidene-1H-[1,2,4]triazole-3,5-diamine
Relevant Products
-
N,N-Dimethyl-p-toluidine
CAS:99-97-8
-
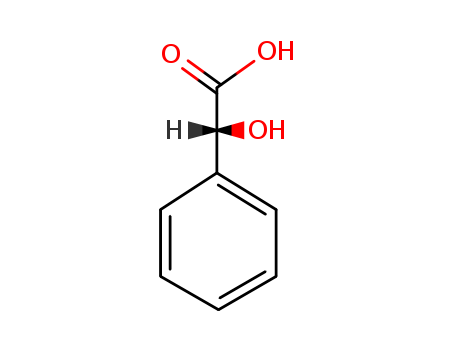
Mandelic acid
CAS:611-71-2
-
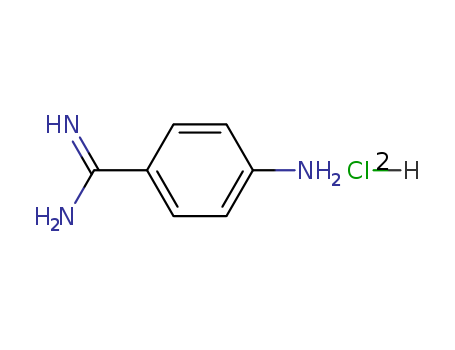
4-Aminobenzamidine dihydrochloride
CAS:2498-50-2