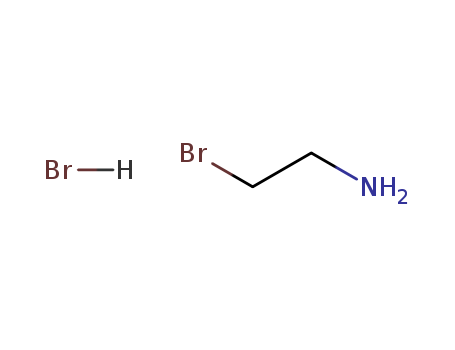
2576-47-8
- Product Name:2-Bromoethylamine hydrobromide
- Molecular Formula:C2H6BrN.HBr
- Purity:99%
- Molecular Weight:204.892
Product Details
Top Quality Chinese Factory supply 2576-47-8 2-Bromoethylamine hydrobromide
- Molecular Formula:C2H6BrN.HBr
- Molecular Weight:204.892
- Appearance/Colour:slight yellow to white crystalline powder
- Vapor Pressure:1.08mmHg at 25°C
- Melting Point:172 °C
- Refractive Index:1.478
- Boiling Point:131.8 °C at 760 mmHg
- Flash Point:33.5 °C
- PSA:26.02000
- Density:1.581g/cm3
- LogP:1.99840
2-Bromoethylamine hydrobromide(Cas 2576-47-8) Usage
|
Preparation |
2-Bromoethylamine hydrobromide was synthesized by bromination of ethanolamine. Drop into hydrobromic acid in the reaction pot, cool, add ethanolamine dropwise under stirring, and finish adding dropwise within half an hour, then steam the hydrobromic acid of 85% of the input quantity (the feed quantity of hydrobromic acid is 8.89 times the weight of ethanolamine) , about 20h steamed. After the concentrated solution is cooled to 70-80°C, it is put into pre-chilled acetone, cooled to below 5°C, and the crystallized 2-bromoethylamine hydrobromide is filtered out. Yield 70%. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
The acidic organic salt (HBr) of the amine. Acidic salts are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: When heated to decomposition 2-Bromoethylamine hydrobromide emits very toxic fumes of bromide ion, NOx and hydrogen bromide. |
|
Fire Hazard |
Flash point data for 2-Bromoethylamine hydrobromide are not available; however, 2-Bromoethylamine hydrobromide is probably combustible. |
|
Application |
2-Bromoethylamine hydrobromide (BEA-HBr) can be used as a reactant in the preparation of:Amino-functionalized ionic liquid, 1-aminoethyl-3-methylimidazolium hexafluorophosphate ([2-aemim][PF6]). [2-aemim][PF6] is employed as a catalyst in the synthesis of 4H-pyrans derivatives by treating with aromatic aldehydes, malononitrile, ethyl acetoacetate via Knoevenagel condensation reaction.2-(N-aryl-N-aroyl)amino-4,5-dihydrothiazole derivatives via cyclocondensation reaction.It can be also employed as an alkylating agent for the surface modification of nylon to obtain primary/secondary/tertiary amine groups. |
|
General Description |
Crystals. |
InChI:InChI=1/C8H16N2/c1-3-6-10(7-4-2)8-5-9/h3-4H,1-2,5-9H2
2576-47-8 Relevant articles
New compound Malabemide, preparation method and uses thereof
-
Paragraph 0024; 0025-0026, (2018/11/03)
The invention discloses a new compound M...
Modifying silica with viologenic groups
Dzaraeva,Arutyuniantz,Archegova,Shpakov
scheme or table, p. 1029 - 1033 (2011/01/06)
Modification of silica with viologenic g...
Studies on the chemistry of aziridine boranes.
Akerfeldt,Wahlberg,Hellstr?m
, p. 115 - 125 (2007/10/16)
-
2576-47-8 Process route
-
-
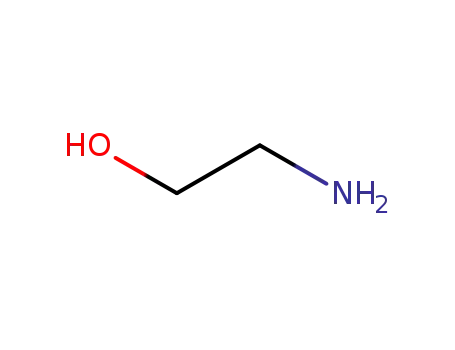
141-43-5
ethanolamine

-
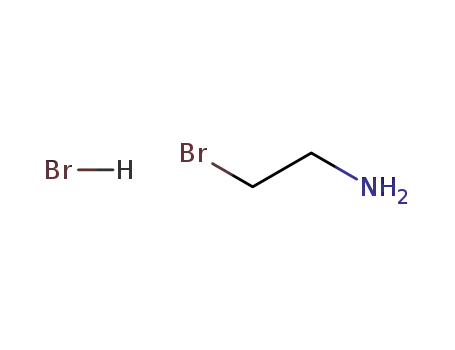
-
2576-47-8
2-bromoethylamine hydrobromide
| Conditions | Yield |
|---|---|
|
With
hydrogen bromide;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 0 - 10 ℃;
for 12h;
|
99% |
|
With
hydrogen bromide;
at 0 ℃;
dann auf 170grad Erhitzen;
|
|
|
With
hydrogen bromide;
Darstellung;
|
|
|
With
hydrogen bromide;
|
-
-
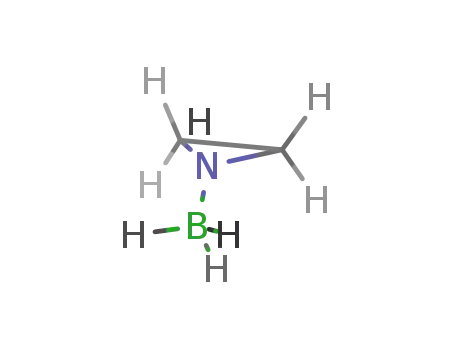
21841-57-6
aziridine borane

-
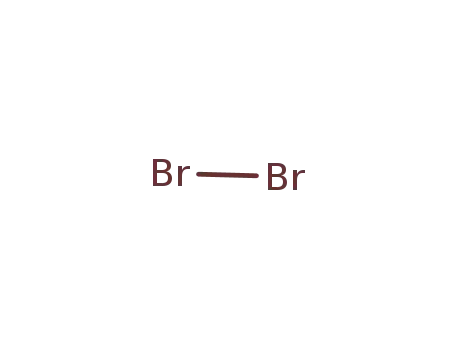
-
7726-95-6
bromine

-
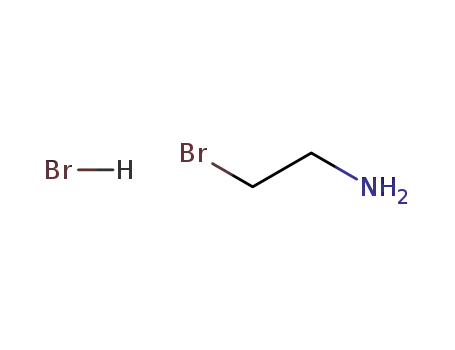
-
2576-47-8
2-bromoethylamine hydrobromide
| Conditions | Yield |
|---|---|
|
In
chloroform;
at 20°C, pptn.; discussion of mechanism;;
|
89% |
|
In
chloroform;
at 20°C, pptn.; discussion of mechanism;;
|
89% |
2576-47-8 Upstream products
-
151-56-4
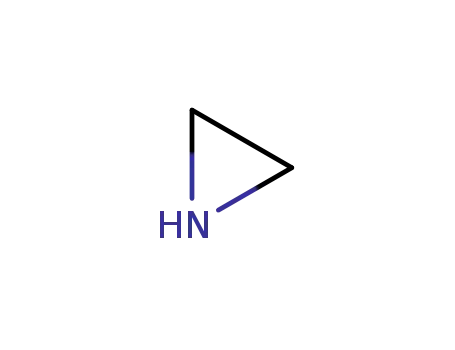
ethyleneimine
-
574-98-1
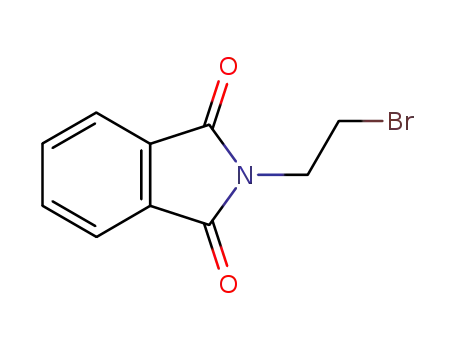
2-(2-bromoethyl)isoindoline-1,3-dione
-
141-43-5

ethanolamine
-
21841-57-6

aziridine borane
2576-47-8 Downstream products
-
111-39-7
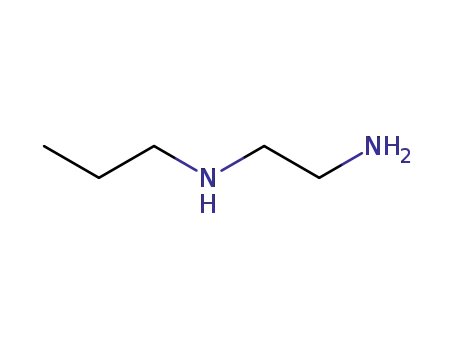
N-propylethane-1,2-diamine
-
16982-46-0
2-ethyl-2-thiazoline
-
14289-10-2
2-pentachlorophenylsulfanyl-ethylamine
-
1483-41-6
1-bromo-2-isothiocyanatoethane
Relevant Products
-
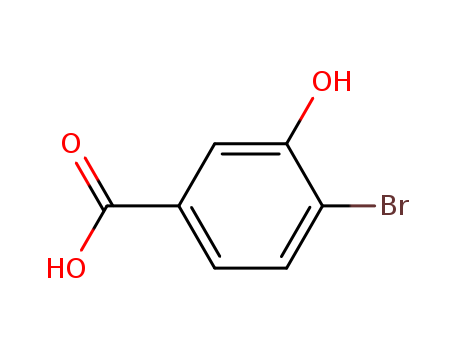
4-BROMO-3-HYDROXYBENZOIC ACID
CAS:14348-38-0
-
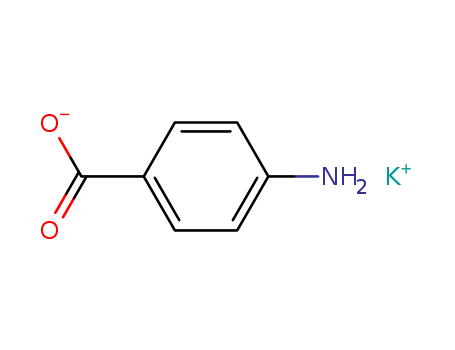
P-AMINOBENZOIC ACID POTASSIUM SALT
CAS:138-84-1
-
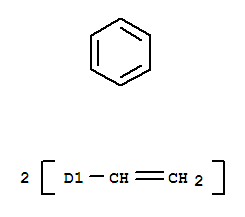
Divinylbenzene
CAS:1321-74-0