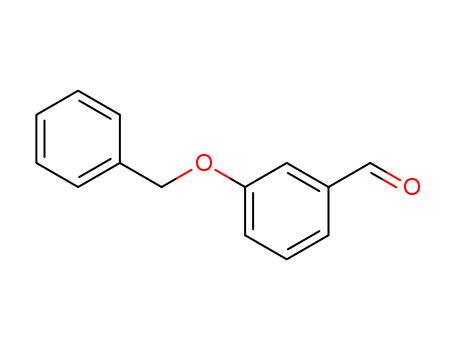
1700-37-4
- Product Name:3-Benzyloxybenzaldehyde
- Molecular Formula:C14H12O2
- Purity:99%
- Molecular Weight:212.248
Product Details
Manufacturer supply 3-Benzyloxybenzaldehyde 1700-37-4 with sufficient stock and high standard
- Molecular Formula:C14H12O2
- Molecular Weight:212.248
- Appearance/Colour:White to light beige fine crystalline powder
- Vapor Pressure:2.54E-05mmHg at 25°C
- Melting Point:56-58 °C(lit.)
- Refractive Index:1.607
- Boiling Point:358.5 °C at 760 mmHg
- Flash Point:164.9 °C
- PSA:26.30000
- Density:1.142 g/cm3
- LogP:3.07810
3-Benzyloxybenzaldehyde(Cas 1700-37-4) Usage
InChI:InChI=1/C14H12O2/c15-10-13-7-4-8-14(9-13)16-11-12-5-2-1-3-6-12/h1-10H,11H2
1700-37-4 Relevant articles
Antitumor agents. Part 3: Synthesis and cytotoxicity of new trans-stilbene benzenesulfonamide derivatives
Yang, Li-Ming,Lin, Shwu-Jiuan,Hsu, Fen-Lin,Yang, Tsang-Hsiung
, p. 1013 - 1015 (2002)
A new series of trans-stilbene benzenesu...
New non-volatile and odorless organosulfur compounds anchored on ionic liquids. Recyclable reagents for Swern oxidation
He, Xun,Chan, Tak Hang
, p. 3389 - 3394 (2006)
A new class of odorless and non-volatile...
Synthesis and anticancer activity of benzyloxybenzaldehyde derivatives against HL-60 cells
Lin, Chin-Fen,Yang, Jai-Sing,Chang, Chiung-Yun,Kuo, Sheng-Chu,Lee, Miau-Rong,Huang, Li-Jiau
, p. 1537 - 1544 (2005)
A series of benzyloxybenzaldehyde deriva...
Design and discovery of silybin analogues as antiproliferative compounds using a ring disjunctive – Based, natural product lead optimization approach
Manivannan, Elangovan,Amawi, Haneen,Hussein, Noor,Karthikeyan, Chandrabose,Fetcenko, Aubry,Narayana Moorthy, N.S. Hari,Trivedi, Piyush,Tiwari, Amit K.
, p. 365 - 378 (2017)
The present study reports the synthesis ...
Benzyloxybenzaldehyde analogues as novel adenylyl cyclase activators
Chang, Chiung-Yun,Kuo, Sheng-Chu,Lin, Yi-Lee,Wang, Jih-Pyang,Huang, Li-Jiau
, p. 1971 - 1974 (2001)
Several benzyloxybenzaldehyde analogues ...
Design, synthesis, biological evaluation and molecular dynamics studies of 4-thiazolinone derivatives as protein tyrosine phosphatase 1B (PTP1B) inhibitors
Liu, Wen-Shan,Wang, Rui-Rui,Yue, Hai,Zheng, Zhi-Hui,Lu, Xin-Hua,Wang, Shu-Qing,Dong, Wei-Li,Wang, Run-Ling
, p. 3814 - 3824 (2020)
Protein tyrosine phosphatase 1B (PTP1B) ...
Synthesis and Structure–Activity Relationships of Novel Benzylamine-Type Antifungals as Butenafine-Related Antimycotics
Krauss, Jürgen,Stadler, Martina,Bracher, Franz
, (2017)
Benzylamine-type antimycotics like nafti...
Computer-Aided Search for 5-Arylideneimidazolone Anticancer Agents Able To Overcome ABCB1-Based Multidrug Resistance
Kaczor, Aneta,Szemerédi, Nikoletta,Kucwaj-Brysz, Katarzyna,D?browska, Monika,Starek, Ma?gorzata,Latacz, Gniewomir,Spengler, Gabriella,Handzlik, Jadwiga
, p. 2386 - 2401 (2021/06/14)
ABCB1 modulation is an interesting strat...
Dibenzazepine-linked isoxazoles: New and potent class of α-glucosidase inhibitors
Umm-E-Farwa,Ullah, Saeed,Khan, Maria Aqeel,Zafar, Humaira,Atia-tul-Wahab,Younus, Munisaa,Choudhary, M. Iqbal,Basha, Fatima Z.
supporting information, (2021/05/10)
α-Glucosidase inhibition is a valid appr...
V2O5@TiO2 Catalyzed Green and Selective Oxidation of Alcohols, Alkylbenzenes and Styrenes to Carbonyls
Upadhyay, Rahul,Kumar, Shashi,Maurya, Sushil K.
, p. 3594 - 3600 (2021/07/02)
The versatile application of different f...
Cerium-photocatalyzed aerobic oxidation of benzylic alcohols to aldehydes and ketones
K?nig, Burkhard,Kumar, Sumit,Stahl, Jessica,Yatham, Veera Reddy,Yedase, Girish Suresh
supporting information, p. 1727 - 1732 (2021/08/05)
We have developed a cerium-photocatalyze...
1700-37-4 Process route
-
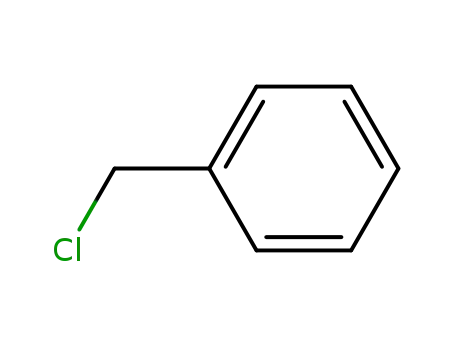
-
100-44-7
benzyl chloride

-
-
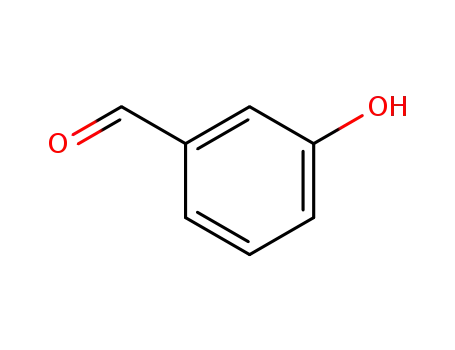
100-83-4
meta-hydroxybenzaldehyde

-
-
1700-37-4
3-Benzyloxybenzaldehyde
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
ethanol;
for 6h;
Reflux;
|
98% |
|
With
potassium carbonate; potassium iodide;
In
acetone;
for 6h;
Heating;
|
92% |
|
With
potassium carbonate; potassium iodide;
In
tetrahydrofuran;
for 6h;
Heating;
|
92% |
|
With
potassium carbonate; sodium iodide;
In
ethanol;
for 4h;
Heating;
|
88% |
|
In
ethanol;
|
88% |
|
With
sodium carbonate;
In
acetone;
|
84% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 50 - 85 ℃;
for 24h;
Inert atmosphere;
|
80% |
|
With
potassium carbonate; potassium iodide;
In
ethanol;
for 4.5h;
Reflux;
|
79% |
|
With
potassium carbonate;
In
ethanol;
for 6h;
Reflux;
|
36% |
|
With
ethanol; potassium carbonate; sodium iodide;
|
|
|
With
potassium carbonate; N,N-dimethyl-formamide;
at 100 ℃;
|
|
|
With
ethanol; sodium ethanolate;
|
|
|
With
sodium hydroxide;
|
|
|
With
hydroxide;
In
water;
|
|
|
With
potassium carbonate; sodium iodide;
In
ethanol;
|
|
|
With
potassium carbonate;
In
ethanol;
Heating;
|
|
|
With
potassium carbonate;
In
ethanol;
Heating;
|
|
|
With
potassium carbonate;
for 4h;
Reflux;
|
|
|
meta-hydroxybenzaldehyde;
With
potassium carbonate;
In
ethanol;
for 0.5h;
Reflux;
benzyl chloride;
In
ethanol;
for 6h;
Reflux;
|
|
|
With
potassium carbonate;
In
acetone;
for 4h;
Reflux;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 55 ℃;
for 3h;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
for 4h;
|
-
-
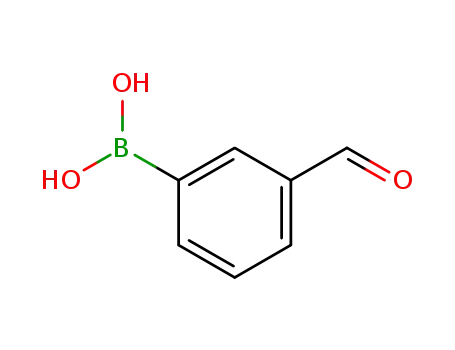
87199-16-4
3-Formylphenylboronic acid

-
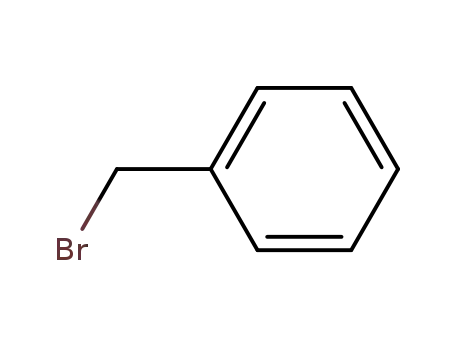
-
100-39-0
benzyl bromide

-

-
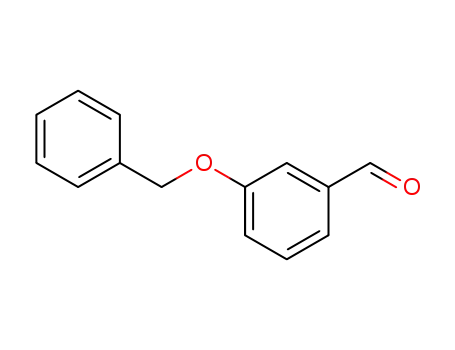
1700-37-4
3-Benzyloxybenzaldehyde
| Conditions | Yield |
|---|---|
|
3-Formylphenylboronic acid;
With
dihydrogen peroxide; cholin hydroxide;
In
water;
at 20 ℃;
for 1h;
Green chemistry;
benzyl bromide;
In
water;
at 20 ℃;
for 2h;
Green chemistry;
|
87% |
1700-37-4 Upstream products
-
100-44-7
benzyl chloride
-
100-83-4

meta-hydroxybenzaldehyde
-
100-39-0
benzyl bromide
-
1700-30-7
(3-(benzyloxy)phenyl)methanol
1700-37-4 Downstream products
-
113697-04-4
4-((Z)-3-benzyloxy-benzylidene)-2-phenyl-4H-oxazol-5-one
-
24550-32-1
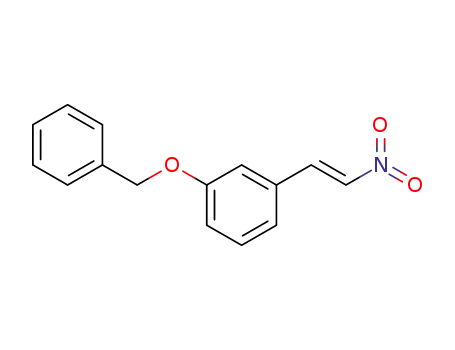
1-(benzyloxy)-3-[(E)-2-nitroethenyl]benzene
-
49646-55-1
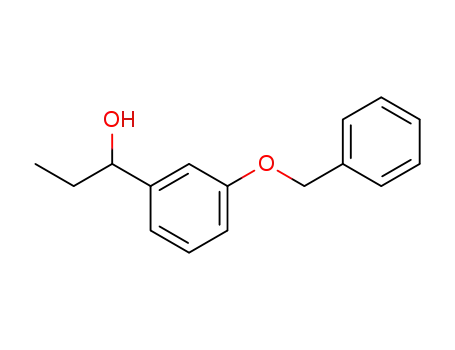
1-(3-(benzyloxy)phenyl)propan-1-ol
-
630-02-4
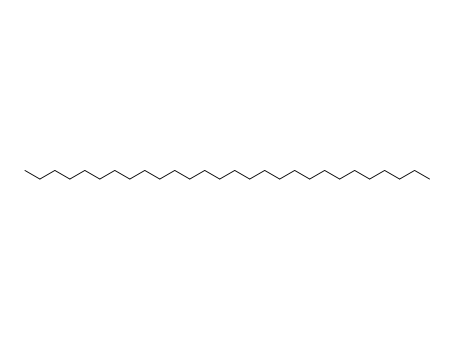
octacosane
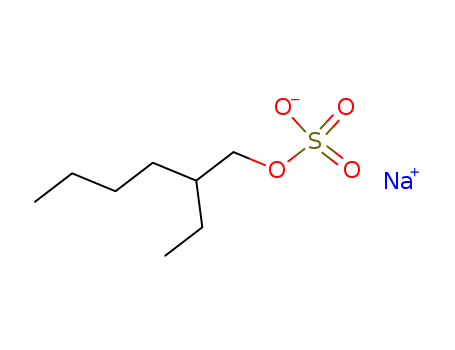
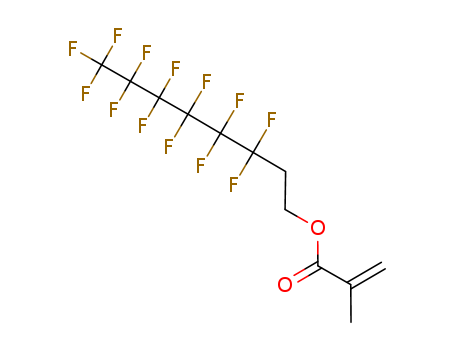
Relevant Products
-
Ethoxylated trimethylolpropane triacrylate
CAS:28961-43-5
-
Sodium 2-ethylhexyl sulfate
CAS:126-92-1
-
2-(Perfluorohexyl)ethyl methacrylate
CAS:2144-53-8