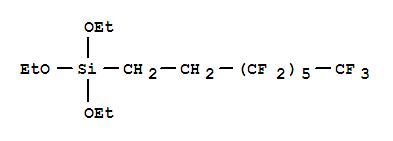
456-22-4
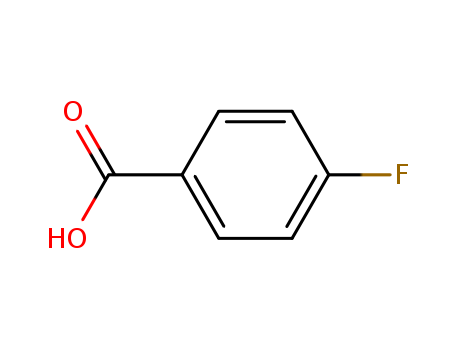
- Product Name:4-Fluorobenzoic acid
- Molecular Formula:C7H5FO2
- Purity:99%
- Molecular Weight:140.114
Product Details
Reliable Quality 4-Fluorobenzoic acid 456-22-4 Hot Sale with Chinese Manufacturer
- Molecular Formula:C7H5FO2
- Molecular Weight:140.114
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00936mmHg at 25°C
- Melting Point:184 °C
- Refractive Index:1.537
- Boiling Point:253.7 °C at 760 mmHg
- PKA:4.15(at 25℃)
- Flash Point:107.2 °C
- PSA:37.30000
- Density:1.319 g/cm3
- LogP:1.52390
4-Fluorobenzoic acid(Cas 456-22-4) Usage
|
Reference |
AG. Vaidyanathan, M. R. Zalutsky, Improved Synthesis of N-Succinimidyl 4-[18F]Fluorobenzoate and Its Application to the Labeling of a Monoclonal Antibody Fragment, Bioconjuate Chemistry, 1994, vol. 5, pp. 352-356 |
|
Purification Methods |
Crystallise the acid from 50% aqueous EtOH, then purify it by zone melting or vacuum sublimation at 130-140o. [Beilstein 9 H 333, 9 III 1327, 9 IV 953.] |
|
Definition |
ChEBI: A fluorobenzoic acid carrying a fluoro substituent at position 4. |
InChI:InChI=1/C7H5FO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)
456-22-4 Relevant articles
Platinum nanoparticles supported on polymeric ionic liquid functionalized magnetic silica: effective and reusable heterogeneous catalysts for the selective oxidation of alcohols in water
Vessally, Esmail,Ghasemisarabbadeih, Mostafa,Ekhteyari, Zeynab,Hosseinzadeh-Khanmiri, Rahim,Ghorbani-Kalhor, Ebrahim,Ejlali, Ladan
, p. 106769 - 106777 (2016)
In this research, the syntheses of core-...
Role of water and p-fluorobenzoic acid in mn(ii)t(p-cl)pp catalyzed aerobic oxidation of p-fluorotoluene
Fang, Yong-Qi,Lv, Chun-Xu,Lu, Ming
, p. 313 - 315 (2013)
A solvent-free manganoporphyrin-catalyze...
New benzothiazole based copper(II) hydrazone Schiff base complexes for selective and environmentally friendly oxidation of benzylic alcohols: The importance of the bimetallic species tuned by the choice of the counterion
Bocian, Aleksandra,Gorczyński, Adam,Marcinkowski, Damian,Witomska, Samanta,Kubicki, Maciej,Mech, Paulina,Bogunia, Ma?gorzata,Brzeski, Jakub,Makowski, Mariusz,Pawlu?, Piotr,Patroniak, Violetta
, (2020)
Green and sustainable chemistry approach...
Polymerization-Enhanced Photosensitization
Wu, Wenbo,Mao, Duo,Xu, Shidang,Kenry,Hu, Fang,Li, Xueqi,Kong, Deling,Liu, Bin
, p. 1937 - 1951 (2018)
Effective photosensitizers are highly de...
Efficient synthesis of aromatic carboxylic acids from aryl ketones in ionic liquid
Jong, Chan Lee,Jang, Mi Lee
, p. 1071 - 1074 (2006)
Conversion of aryl ketones to the corres...
Synthesis and evaluation of indole-substituted N-heterocyclic carbene ligands
Yan, Huan,Liu, Zhongxian,Tan, Kai,Ji, Ruigeng,Ye, Yingxin,Yan, Tingbing,Shen, Yuehai
, (2020)
Indole-substituted N-heterocyclic carben...
Active-sodium-promoted reductive cleavage of halogenated benzoic acids
Azzena, Ugo,Dettori, Giovanna,Mocci, Sarah,Pisano, Luisa,Cerioni, Giovanni,Mocci, Francesca
, p. 9171 - 9174 (2010)
The outcome of the reaction between 1,2-...
Transformation of Thioacids into Carboxylic Acids via a Visible-Light-Promoted Atomic Substitution Process
Fu, Qiang,Liang, Fu-Shun,Lou, Da-Wei,Pan, Gao-Feng,Wang, Rui,Wu, Min,Xie, Kai-Jun
supporting information, p. 2020 - 2024 (2022/03/31)
A visible-light-promoted atomic substitu...
Mechanochemical Grignard Reactions with Gaseous CO2 and Sodium Methyl Carbonate**
Pfennig, Victoria S.,Villella, Romina C.,Nikodemus, Julia,Bolm, Carsten
supporting information, (2022/01/22)
A one-pot, three-step protocol for the p...
Scrutinizing ligand exchange reactions in the formation of the precious group metal-organic framework RuII,II-HKUST-1: The impact of diruthenium tetracarboxylate precursor and modulator choice
Heinz, Werner R.,Staude, Dominik,Mayer, David,Bunzen, Hana,Fischer, Roland A.
supporting information, p. 5226 - 5235 (2021/04/26)
The precious group metal (PGM) analogues...
Oxidative carbon-carbon bond cleavage of 1,2-diols to carboxylic acids/ketones by an inorganic-ligand supported iron catalyst
Chen, Weiming,Xie, Xin,Zhang, Jian,Qu, Jian,Luo, Can,Lai, Yaozhu,Jiang, Feng,Yu, Han,Wei, Yongge
supporting information, p. 9140 - 9146 (2021/11/23)
The carbon-carbon bond cleavage of 1,2-d...
456-22-4 Process route
-
-
2729-19-3
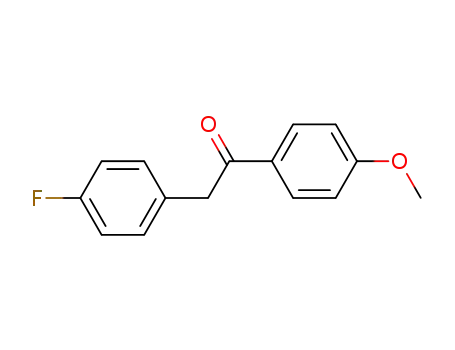
2-(4-Fluorophenyl)-1-(4-methoxyphenyl)ethan-1-one

-
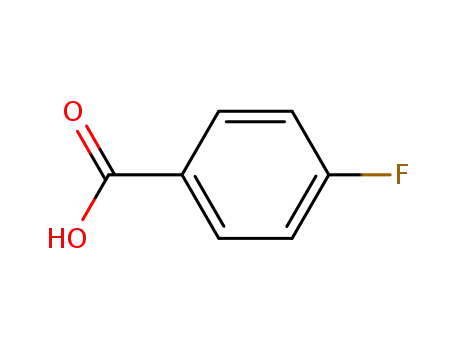
-
456-22-4
4-Fluorobenzoic acid

-
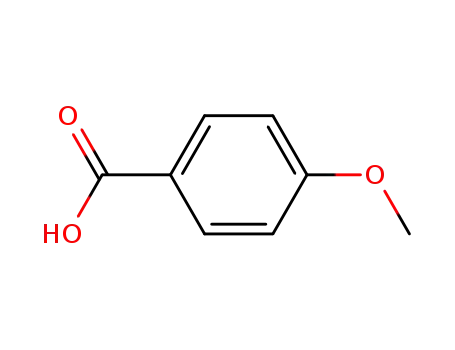
-
100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; potassium hydroxide;
at 20 ℃;
chemoselective reaction;
Schlenk technique;
|
96% 95% |
-
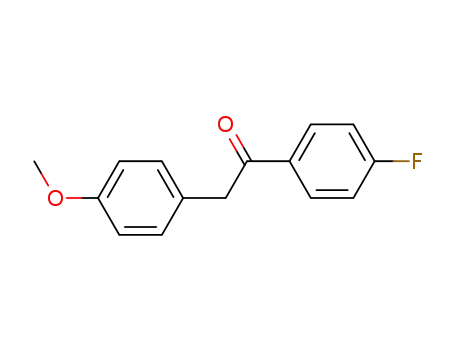
-
2729-18-2
1-(4-fluorophenyl)-2-(4-methoxyphenyl)ethan-1-one

-

-
456-22-4
4-Fluorobenzoic acid

-

-
100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
oxygen; potassium hydroxide;
at 20 ℃;
chemoselective reaction;
Schlenk technique;
|
96% 94% |
456-22-4 Upstream products
-
595-07-3
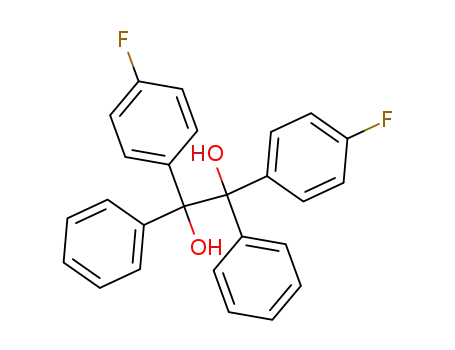
1,2-bis-(4-fluoro-phenyl)-1,2-diphenyl-ethane-1,2-diol
-
75-36-5
acetyl chloride
-
94-09-7
p-aminoethylbenzoate
-
65972-02-3
N-Trimethylammonio-p-fluorbenzamidat
456-22-4 Downstream products
-
451-46-7
4-fluorobenzoic acid ethyl ester
-
459-56-3
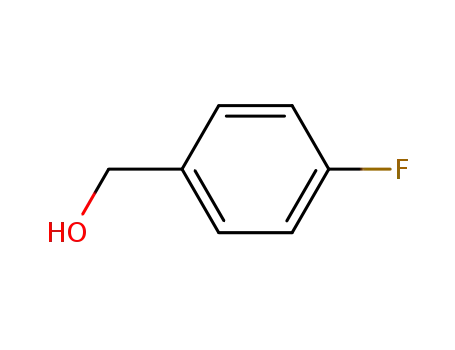
4-fluorobenzylic alcohol
-
403-33-8
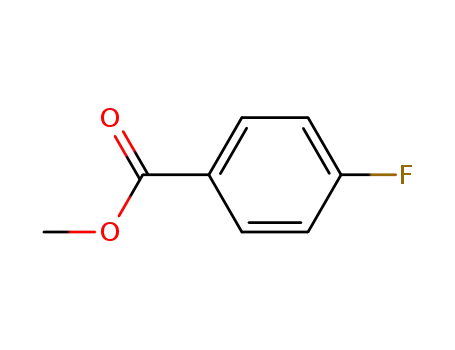
methyl 4-flurobenzoate
-
453-71-4
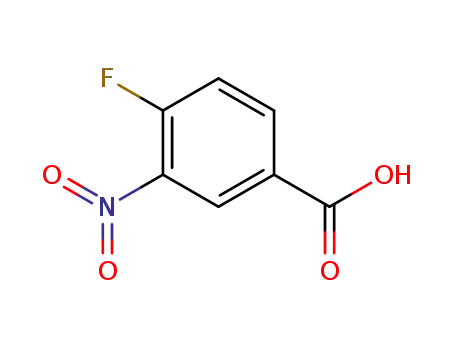
3-nitro-4-fluorobenzoic acid
Relevant Products
-
1H,1H,2H,2H-Perfluorooctyltriethoxysilane
CAS:51851-37-7
-
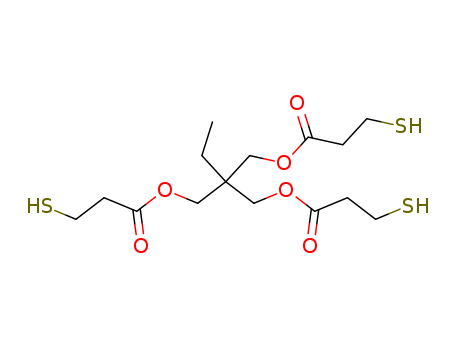
Trimethylolpropane Tris(3-mercaptopropionate)
CAS:33007-83-9
-
Octadecanethiol
CAS:2885-00-9