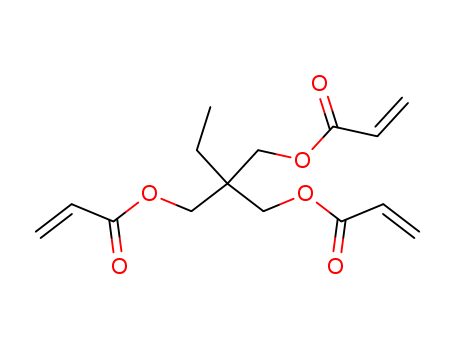
15625-89-5
- Product Name:Trimethylolpropane triacrylate
- Molecular Formula:C15H20O6
- Purity:99%
- Molecular Weight:296.32
Product Details
Quality Manufacturer Supply High Purity 99% Trimethylolpropane triacrylate 15625-89-5 with Reasonable Price
- Molecular Formula:C15H20O6
- Molecular Weight:296.32
- Appearance/Colour:Pale yellow to yellow transparent liquid
- Vapor Pressure:<0.01 mm Hg ( 20 °C)
- Melting Point:-66 °C
- Refractive Index:n20/D 1.474(lit.)
- Boiling Point:380.9 °C at 760 mmHg
- PKA:0[at 20 ℃]
- Flash Point:165 °C
- PSA:78.90000
- Density:1.089 g/cm3
- LogP:1.57040
Trimethylolpropane triacrylate(Cas 15625-89-5) Usage
|
Preparation |
Synthesis of trimethylolpropane triacrylate: 2.44 kg of trimethylolpropane, 3.93 kg of acrylic acid, 0.5 kg of an acid ion exchanger (Lewatit 3333 of BAYER AG), 9 g of hydroquinone and 1.5 liters of petroleum ether (boiling range 60° to 70° C) were heated under a water separator, whilst stirring, in a 10 liter three-necked flask equipped with a stirrer, water separator and gas inlet tube. At the same time, a constant stream of air saturated with allyl alcohol was passed through the flask at a rate of 2 liters/hour. In total, 16 ml of allyl alcohol were introduced into the esterification mixture in this way. After 80 hours, the reaction mixture had an acid number of 32 and the boiling point had risen to 72° C. The petroleum ether was distilled off, remnants being removed by applying a vacuum of 0.2 mm Hg at a product temperature of 40° C. The esterification catalyst was separated off by filtration and was kept for further use. The trimethylolpropane triacrylate obtained in practically quantitative yield was pale yellowish and clear. The viscosity corresponded to a time of outflow of 20 seconds measured in a DIN cup 4 at 20° C. The product showed no change even on storage for 4 months at 60° C. When used in a UV light-curing printing ink which contained a photoinitiator, the product polymerised at high speed.Literature source US04059721 |
|
Flammability and Explosibility |
Notclassified |
InChI:InChI=1/C15H20O9/c16-8-15(9-17,10-18)7-14(4-1-11(19)20,5-2-12(21)22)6-3-13(23)24/h1-6,16-18H,7-10H2,(H,19,20)(H,21,22)(H,23,24)/p-3/b4-1+,5-2+,6-3+
15625-89-5 Relevant articles
Synthesis and characterization of novel ionophores of double-armed penta-crown ethers
Zhi, Bin Huang,Seung, Hyun Chang
, p. 5351 - 5355 (2005)
Unique structures of novel ionophores of...
Synthesis of novel tris-crown ether structures
Huang, Zhi Bin,Chang, Seung Hyun
, p. 1703 - 1706 (2005)
Six unique tris-crown ether structures w...
Synthesis method of trimethylolpropane triacrylate
-
Paragraph 0021-0042, (2020/07/12)
The invention provides a synthesis metho...
Preparation method of trimethylolpropane triacrylate
-
Paragraph 0010; 0011; 0012; 0013; 0014; 0015, (2017/07/11)
Compared with the prior art, a preparati...
Chemoselective Transesterification of Acrylate Derivatives for Functionalized Monomer Synthesis Using a Hard Zinc Alkoxide Generation Strategy
Nakatake, Daiki,Yazaki, Ryo,Ohshima, Takashi
supporting information, p. 3696 - 3699 (2016/08/20)
A new practical method for the synthesis...
15625-89-5 Process route
-
-
77-99-6
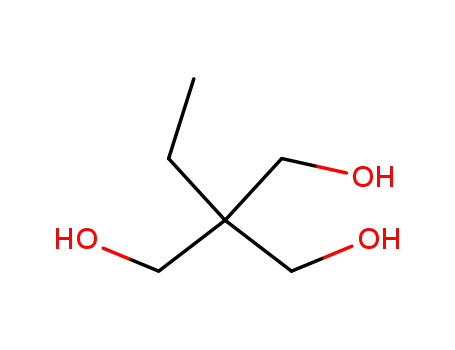
1,1,1-tri(hydroxymethyl)propane

-
-
79-10-7
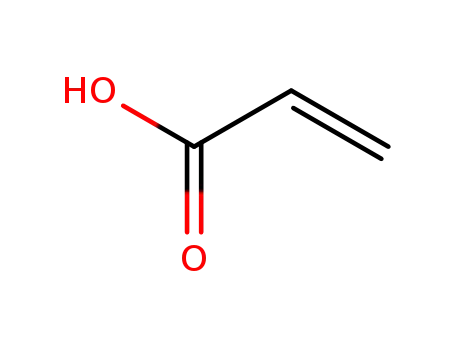
acrylic acid

-
-
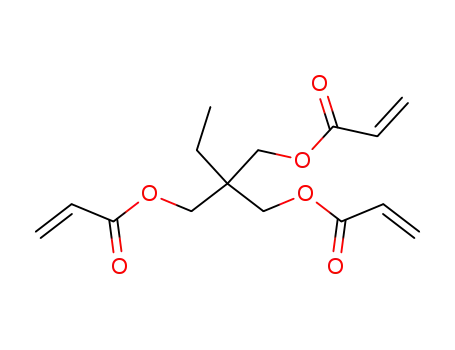
15625-89-5
2-((acryloyloxy)methyl)-2-ethylpropane-1,3-diyl diacrylate
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid; hypophosphorous acid; hydroquinone;
In
cyclohexane;
at 70 - 91 ℃;
under 3750.38 Torr;
|
95% |
|
With
hypophosphorous acid; 4-methoxy-phenol; copper dichloride;
In
cyclohexane; water;
at 70 - 75 ℃;
1,1,1-tri(hydroxymethyl)propane; acrylic acid;
With
toluene-4-sulfonic acid;
In
cyclohexane; water;
at 95 - 130 ℃;
for 6 - 10h;
|
|
|
With
methanesulfonic acid; hydroquinone;
In
toluene;
at 120 ℃;
for 5h;
|
-

-
77-99-6
1,1,1-tri(hydroxymethyl)propane

-
-
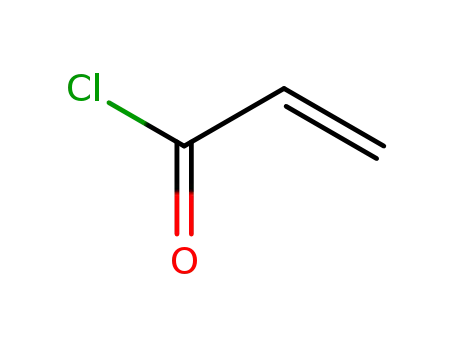
814-68-6,25189-84-8
acryloyl chloride

-

-
15625-89-5
2-((acryloyloxy)methyl)-2-ethylpropane-1,3-diyl diacrylate
| Conditions | Yield |
|---|---|
|
With
4-methoxy-phenol; triethylamine; hydroquinone;
at 60 ℃;
for 2h;
Temperature;
|
98.38% |
|
In
pyridine; benzene;
at 50 ℃;
for 30h;
|
81% |
|
With
pyridine;
In
benzene;
at 50 ℃;
for 30h;
|
81% |
15625-89-5 Upstream products
-
77-99-6

1,1,1-tri(hydroxymethyl)propane
-
814-68-6

acryloyl chloride
-
79-10-7

acrylic acid
-
37275-47-1
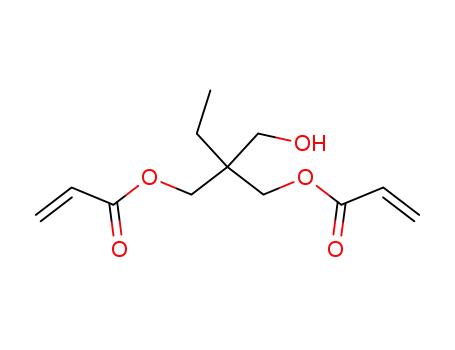
trimethylolpropane diacrylate
15625-89-5 Downstream products
-
107-15-3
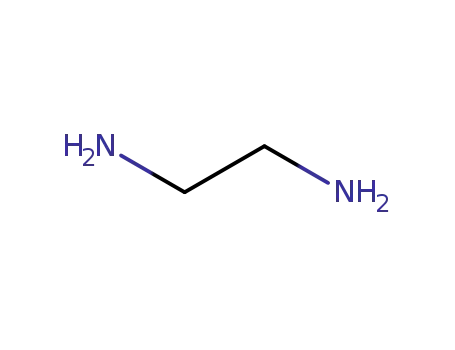
ethylenediamine
-
1337537-13-9
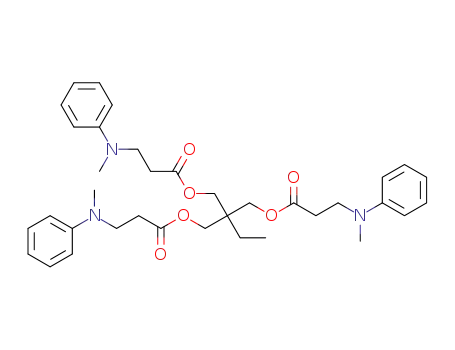
1,1,1-trimethylolpropane tri-(3-[N-methyl-N-phenylamino]propionate)
-
1337537-26-4
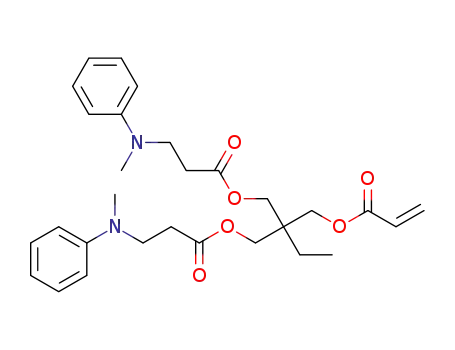
C29H38N2O6
-
1337537-29-7
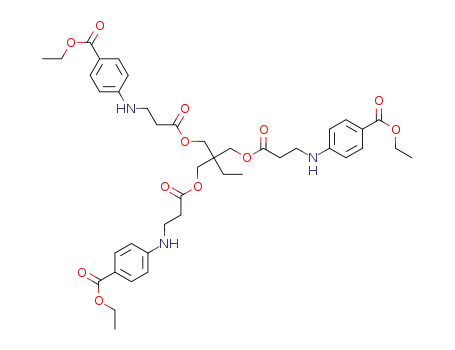
C42H53N3O12
Relevant Products
-
Ethoxylated trimethylolpropane triacrylate
CAS:28961-43-5
-
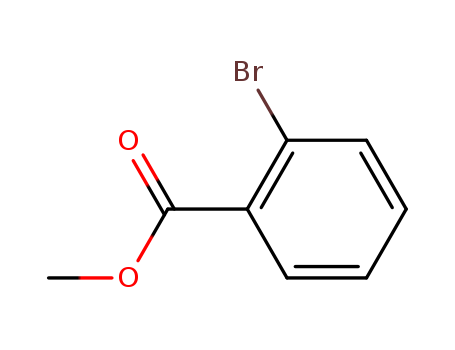
Methyl 2-bromobenzoate
CAS:610-94-6
-
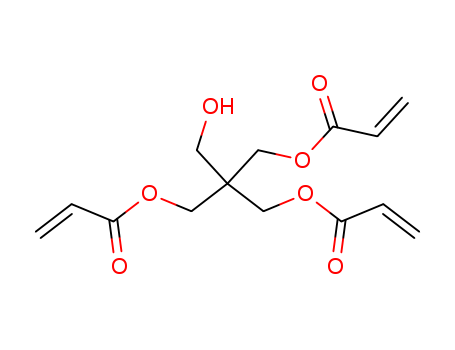
Pentaerythritol triacrylate
CAS:3524-68-3