623-05-2
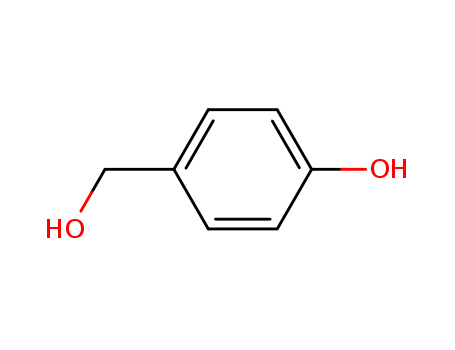
- Product Name:4-Hydroxybenzyl alcohol
- Molecular Formula:C7H8O2
- Purity:99%
- Molecular Weight:124.139
Product Details
Manufacturer Supply Best Quality 4-Hydroxybenzyl alcohol 623-05-2 with Efficient Transportation
- Molecular Formula:C7H8O2
- Molecular Weight:124.139
- Appearance/Colour:Pink to beige crystalline powder
- Vapor Pressure:0.0104mmHg at 25°C
- Melting Point:110-112 °C
- Refractive Index:1.595
- Boiling Point:252 °C at 760 mmHg
- PKA:pK1:9.82 (25°C)
- Flash Point:145.8 °C
- PSA:40.46000
- Density:1.22 g/cm3
- LogP:0.88450
4-Hydroxybenzyl alcohol(Cas 623-05-2) Usage
|
Preparation |
Prepared from p-cresol by using the microorganism, Pseudomonas putida. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the alcohol from H2O, *C6H6 (m 124o), *C6H6/EtOH or ClCH2CH2Cl (m 122o). [Beilstein 6 III 4546, 6 IV 5909.] |
|
Definition |
ChEBI: A member of the class of benzyl alcohols that is benzyl alcohol substituted by a hydroxy group at position 4. It has been isolated from Arcangelisia gusanlung. |
|
General Description |
4-Hydroxybenzyl alcohol (HBA) is a phenolic compound found in Gastrodia elata. It is as an antioxidant and anti-asthmatic agent. |
InChI:InChI=1/C7H8O2/c8-5-6-1-3-7(9)4-2-6/h1-4,8-9H,5H2
623-05-2 Relevant articles
Acute toxicity of benzoic acids to the crustacean Daphnia magna
Kamaya, Yasushi,Fukaya, Yuki,Suzuki, Kyoji
, p. 255 - 261 (2005)
The acute immobilization toxicity of ben...
Selective Synthesis of 4-Hydroxymethylphenol catalysed by Cyclodextrins having Hydroxypropyl Residues
Komiyama, Makoto
, p. 651 - 652 (1988)
The selective synthesis of 4-hydroxymeth...
-
Hutchinson
, (1891)
-
Determining Factors for the Product Para/Ortho Ratio and Reaction Rate in the Formation of (Hydroxymethyl)phenols from Phenol and Formaldehyde
Komiyama, Makoto
, p. 2079 - 2082 (1988)
Formations of 2- and 4-(hydroxymethyl)ph...
Discovery of new and highly effective quadruple FFA1 and PPARα/γ/δ agonists as potential anti-fatty liver agents
Cai, Zongyu,Deng, Liming,Geng, Xinqian,Hu, Lijun,Jiao, Shixuan,Li, Zheng,Ren, Qiang,Wang, Bin,Yang, Ying,Zhang, Luyong,Zhou, Zongtao
supporting information, (2021/12/27)
Non-alcoholic fatty liver disease (NAFLD...
Investigation of the effect of different linker chemotypes on the inhibition of histone deacetylases (HDACs)
Linciano, Pasquale,Benedetti, Rosaria,Pinzi, Luca,Russo, Fabiana,Chianese, Ugo,Sorbi, Claudia,Altucci, Lucia,Rastelli, Giulio,Brasili, Livio,Franchini, Silvia
, (2020/11/24)
Histone Deacetylases (HDACs) are among t...
Hydroboration Reaction and Mechanism of Carboxylic Acids using NaNH2(BH3)2, a Hydroboration Reagent with Reducing Capability between NaBH4and LiAlH4
Wang, Jin,Ju, Ming-Yue,Wang, Xinghua,Ma, Yan-Na,Wei, Donghui,Chen, Xuenian
, p. 5305 - 5316 (2021/04/12)
Hydroboration reactions of carboxylic ac...
Ambient-pressure highly active hydrogenation of ketones and aldehydes catalyzed by a metal-ligand bifunctional iridium catalyst under base-free conditions in water
Wang, Rongzhou,Yue, Yuancheng,Qi, Jipeng,Liu, Shiyuan,Song, Ao,Zhuo, Shuping,Xing, Ling-Bao
, p. 1 - 7 (2021/05/17)
A green, efficient, and high active cata...
623-05-2 Process route
-
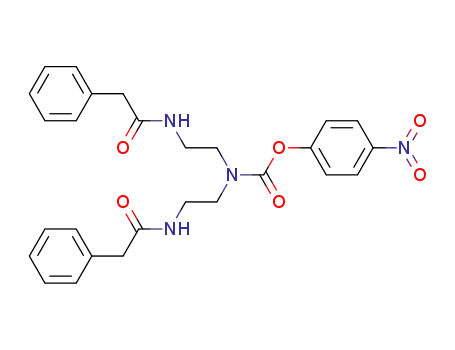
-
757967-03-6
bis-(2-phenylacetylamino-ethyl)-carbamic acid 4-nitro-phenyl ester

-
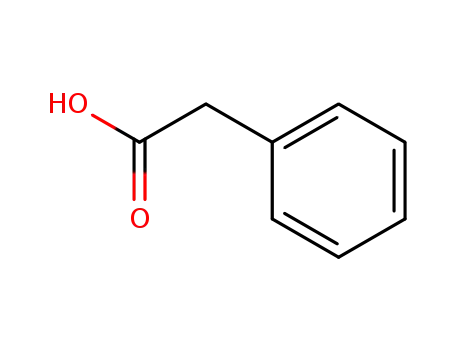
-
103-82-2
phenylacetic acid

-
-
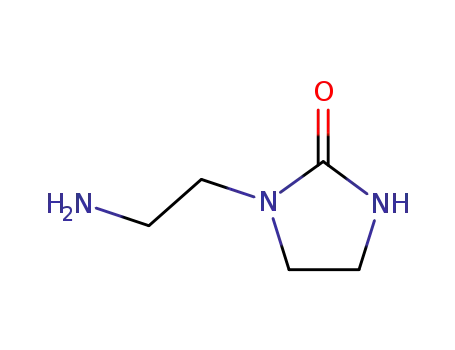
6281-42-1
2-aminoethylimidazolidone

-
-
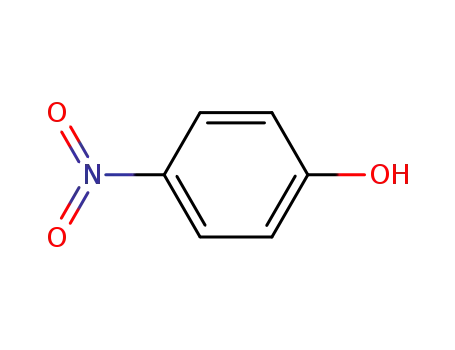
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
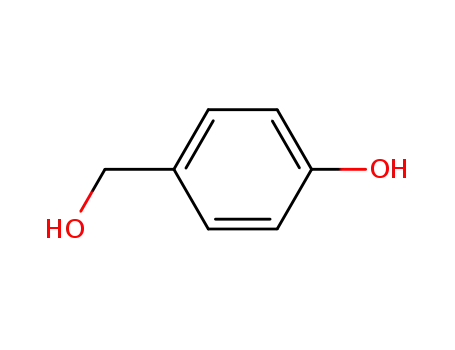
623-05-2
(4-hydroxyphenyl)methanol

-
-
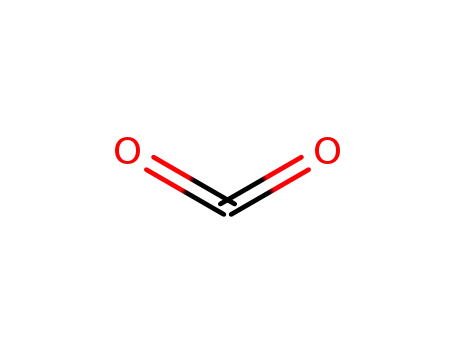
124-38-9,18923-20-1
carbon dioxide
| Conditions | Yield |
|---|---|
|
With
water;
penicillin-G-amidase;
In
Chremephor EL; dimethyl sulfoxide;
at 37 ℃;
pH=7.4;
Kinetics;
Enzymatic reaction;
Aqueous phosphate buffer;
|
-
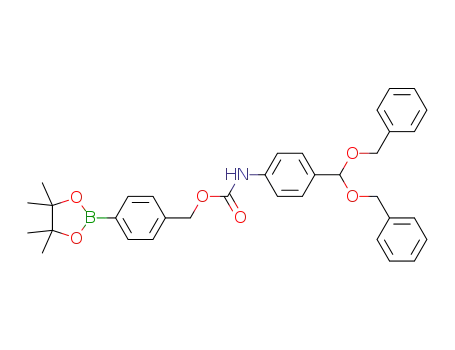
-
1349832-78-5
4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl 4-(bis(benzyloxy)methyl)phenylcarbamate

-
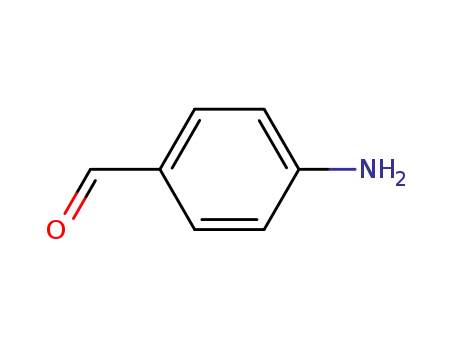
-
556-18-3,28107-09-7
4-aminobenzaldehyde

-

-
623-05-2
(4-hydroxyphenyl)methanol

-
-
1286727-45-4
C14H13NO2

-
-
1286727-47-6
C21H19NO3

-
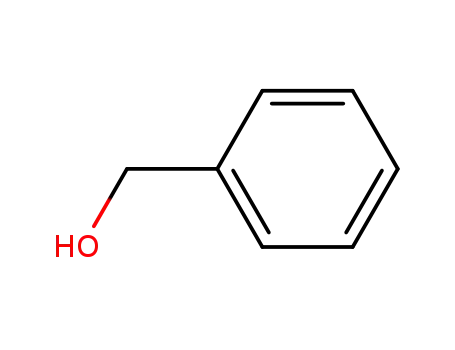
-
100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With
urea hydrogen peroxide adduct;
In
water; acetonitrile;
at 25 ℃;
for 3h;
pH=8;
aq. phosphate buffer;
|
623-05-2 Upstream products
-
120-47-8
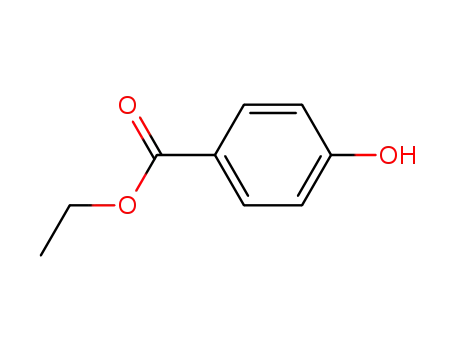
Ethyl 4-hydroxybenzoate
-
836-43-1
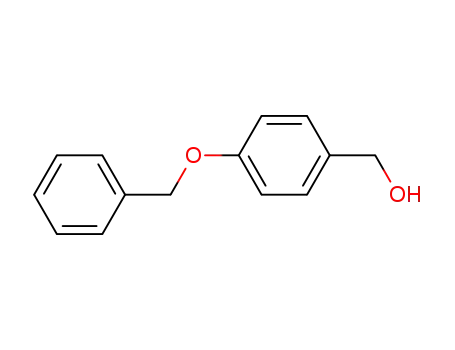
4-benzyloxybenzyl alcohol
-
2316-62-3
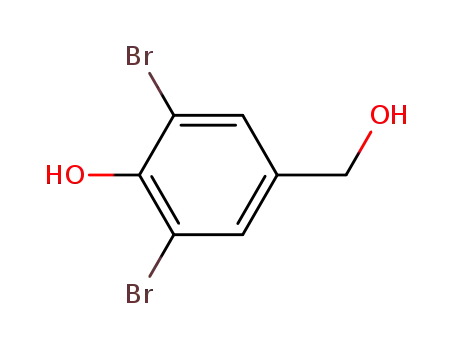
3,5-dibromo-4-hydroxybenzyl alcohol
-
50-00-0
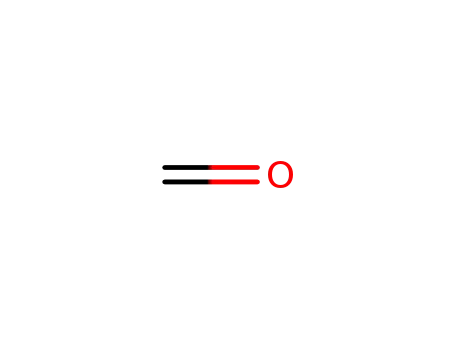
formaldehyd
623-05-2 Downstream products
-
5355-17-9
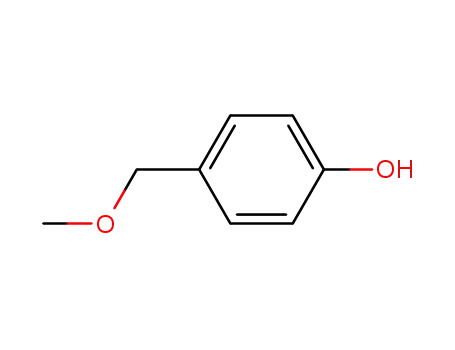
4-hydroxybenzyl methyl ether
-
2937-61-3
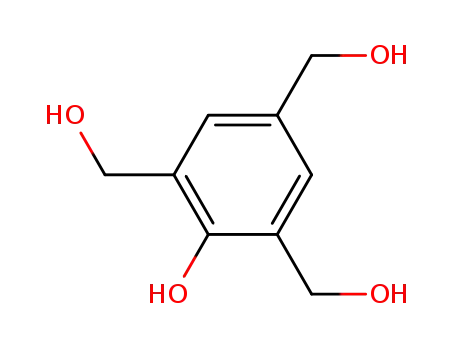
2,4,6-tris(hydroxymethyl)phenol
-
57726-26-8
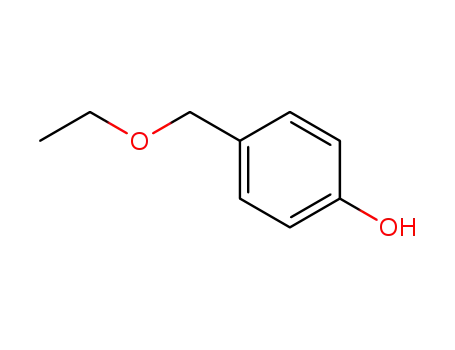
4-hydroxybenzyl ethyl ether
-
2937-64-6
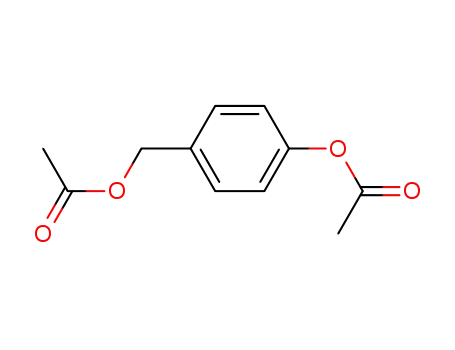
4-(acetyloxy)benzyl acetate
Relevant Products
-
Ethoxylated trimethylolpropane triacrylate
CAS:28961-43-5
-
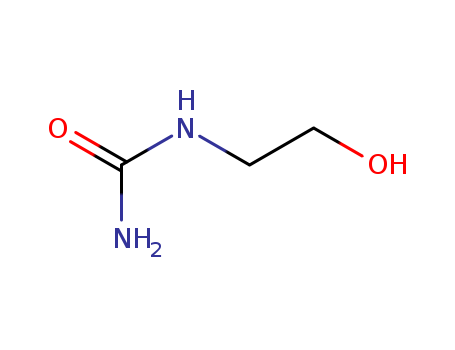
2-HYDROXYETHYLUREA
CAS:2078-71-9
-
Dodecyldimethylbenzylammonium chloride
CAS:139-07-1